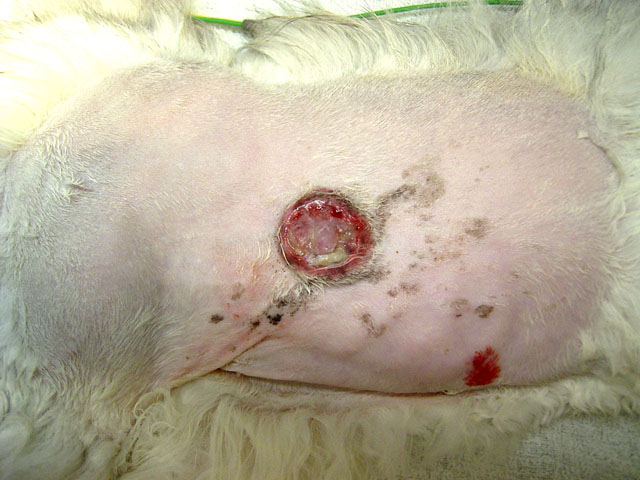
Signalment:
Gross Description:
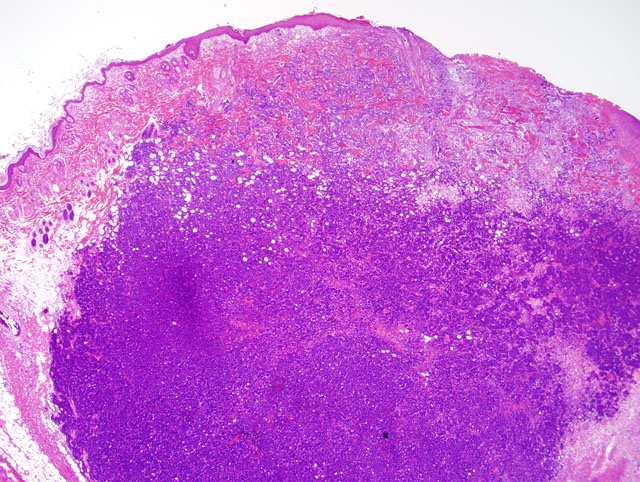
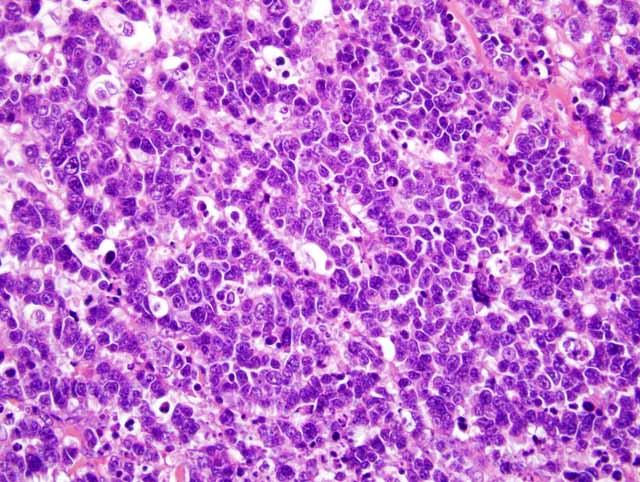
Histopathologic Description:
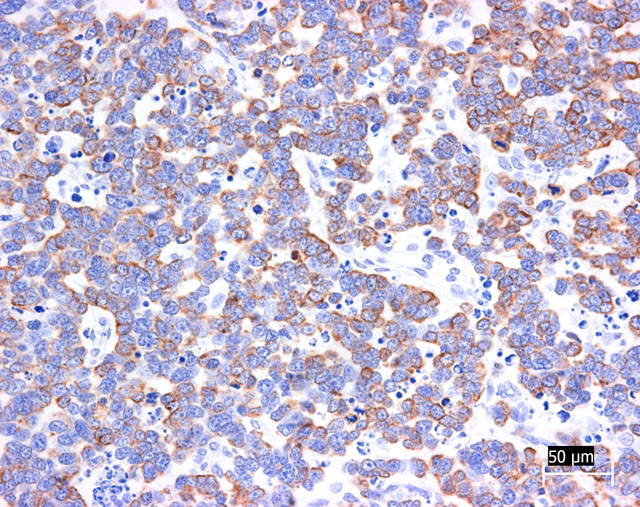
Grimelius reaction for argyrophilic granules was positive in tumor cells, because they often showed small dark granules in cytoplasm. Immunohistochemically, almost all tumor cells were positive for cytokeratin 20 and synaptophysin (Fig. 3-4). The positivity for cytokeratin 20 has a perinuclear dot-like structure (Fig.5). Tumor cells have shown focal reactivity for chromogranin A and PGP9.5. However, no positive reaction was observed for cytokeratin 8/18, AE1/AE3, S100, CD3, CD20, and vimentin.
Morphologic Diagnosis:
Condition:
Contributor Comment:
Trichoblastoma and sweat gland tumor was most difficult to differentiate from Merkel cell carcinoma. In our case, gland-like structure and intracytoplasmic cyst suggested sweat gland origin of tumor cells. However, morphologic pattern of immunohistochemical positivity for cytokeratin 20 was very characteristic and suggestive of Merkel cell tumor. Recently, it was reported that immunohistochemical examination using cytokeratin 20 is extremely useful in distinguishing Merkel cell tumor from trichoblastoma and sweat gland tumor.(3) According to this report, Merkel cell tumor was characterized by perinuclear dot-like structure (keratin button) for cytokeratin 20 like those in the present case. In addition, negative staining for cytokeratin 8/18 was able to deny sweat gland differentiation. Trichoblastoma often included focal positivity for synaptophysin and chromogranin A, indicating neuroendocrine differentiation. However, in our case, many tumor cells were positive for synaptophysin and focal positivity for PGP9.5 was seen. Therefore, our case was derived from neuroendocrine cells.
In humans, small cell carcinoma of lung often metastasizes to the skin. The growth pattern of that tumor resembles Merkel cell tumor. Cytokeratin 20 was usually negative in small cell carcinoma, but there was no report in animals.(3) In our case, as necropsy was not performed, metastatic tumor could not be completely denied. However, positivity for cytokeratin 20 strongly suggested Merkel cell origin.
Only two cases of Merkel cell carcinoma of cats have been reported.(1,4) One case was a relapse and pulmonary metastasis, but another case showed no relapse and metastasis. In our case, lymph node metastasis and multiple mitotic figures indicated malignant behavior of this tumor. In dogs, Merkel cell tumor was benign, but in humans it was malignant.(2) Therefore, clinical behavior of cats was similar to that of humans.Â
JPC Diagnosis:
Conference Comment:
Debate over the origin of Merkel cells continues, and it is now commonly thought that these cells arise from the neural crest. Immunhistochemical staining helps support this theory because Merkel cells stain for neuron-specific enolase, chromogranin A, and synaptophysin. As the contributor mentioned, Merkel cells are also positive for CK 20, and they are negative for CD45 and CD18 ruling out a leukocyte origin. Rare cases of Merkel cell tumors have been reported in dogs, and this is only the third reported case in a cat. Merkel cell tumors are normally intradermal, unencapsulated tumors composed of solid, dense nests and packets separated and supported by a fine fibrovascular stroma. There is normally little cellular atypia, and mitotic figures are rare. Ultrastructurally, Merkel cells have characteristic membrane-bound dense core granules and rudimentary desmosomal structures.(3)
Dr. Goldschmidt pointed out an area adjacent to the neoplasm of abrupt transition from normal epidermis to an area of flat raised acanthotic epidermis (not evident in all sections) strongly suggestive of viral plaque. Pathologists in the AFIP Department of Soft Tissue Pathology reviewed this case and concurred with the diagnosis of Merkel cell carcinoma.
References:
2. Konno A, Nagata M, Nanko H: Immunohistochemical diagnosis of a Merkel cell tumor in a dog. Vet Pathol 35:538-540, 1998
3. Moll R, Lowe A, Laufer J, Franke WW: Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol 140:427-447, 1992
4. Patnaik AK, Post GS, Erlandson RA: Clinicopathologic and electron microscopic study of cutaneous neuroendocrine (Merkel cell) carcinoma in a cat with comparisons to human and canine tumors. Vet Pathol 38:553-556, 2001