Signalment:
Gross Description:
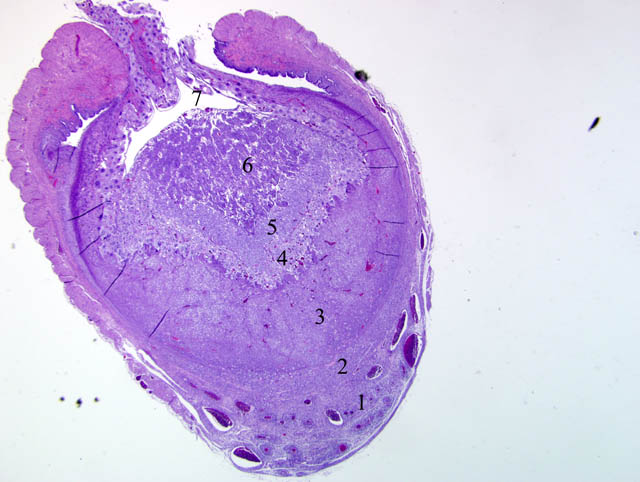
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Decidual alteration was referred to as deciduoma in earlier lab exploratory pathology literature because the lesion was originally regarded as neoplastic.(5) Instead, this is a proliferative, well organized structure that generally follows a predictable time-course. The alteration can be induced between days 3 and 4 of pseudopregnancy, and requires priming or sensitization of the uterus by progesterone for at least 48 hours, followed by minute amounts of estrogen.(4) Most often, this sequence of events is linked to the late luteal phase of the estrus cycle.(5,6) Decidual alteration in the rat has also been reported following various forms of mechanical irritation or trauma, electrical stimulation, intrauterine instillation of agents such as sesame oil, Hanks balanced salt solution, histamine, air, or prostaglandins, as well as administration of progestin, such as 19-norpregnane.(4,5) The alteration will generally undergo regression between days 12-16 (nodular form) and 21 (diffuse form) of pseudopregnancy, with necrosis and cleavage of the capsule from the underlying uterine wall.(4)
Decidual alteration occurs spontaneously in rats, usually at a low incidence (<1% over a 10 year period in one study).(4) The incidence in this group of young adult control rats was unusual in our experience. According to Elcock et al., vitamin E deficiency may be a contributing factor to increased spontaneous incidence of deciduoma, with 60% of rats fed vitamin E deficient diets developing spontaneous deciduoma compared with 4% on natural food.(4)
JPC Diagnosis:
Conference Comment:
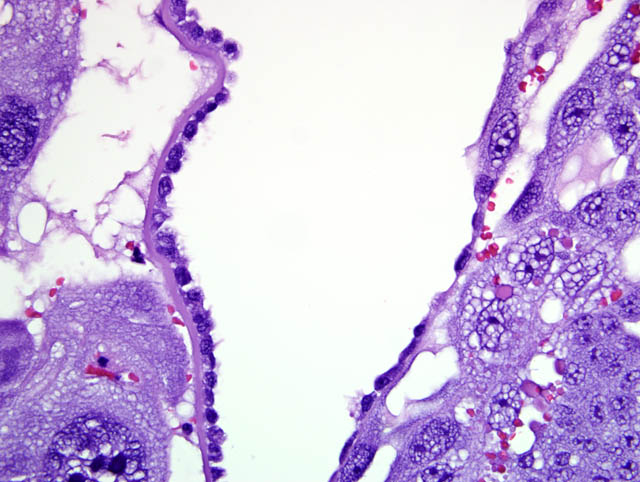
Although participants recognized exuberant decidualization in the mesometrial aspect of the uterus, characterized by the ubiquitous presence of large, pale, glycogen-rich decidual cells in the myometrium and stroma, many could not discern the six components of a classic deciduoma, i.e. metrial gland in the myometrium on the mesometrial side of the uterus, basal zone separating the deciduoma from the myometrium, a capsule just luminal to the basal zone, mesometrial and antimesometrial regions, and a glycogenic transitional area.(6) Rather, participants noted five distinct layers in the mesometrial aspect of the uterus that, collectively, are interpreted as structures of a placental disk. Notably, the characteristic layers are not described as part of a deciduoma. From adluminal to luminal, they are:
- Decidualized myometrium: The mesometrial myometrium is composed of large decidual cells with abundant, slightly basophilic, vacuolated cytoplasm that often contains PAS-positive granules, round nuclei and one or two distinct nucleoli. Larger vessels within the decidualized myometrium are surrounded by numerous metrial cells, and the endothelium is often covered by large cytotrophoblasts.
- Decidualized stromal layer: Separated from the decidualized myometrium by a discontinuous, indistinct, thin band of myometrial smooth muscle cells is a completely decidualized stromal layer; this layer is composed of decidual cells as described in the myometrium, with clear cytoplasmic vacuoles that become progressively prominent toward the luminal surface.
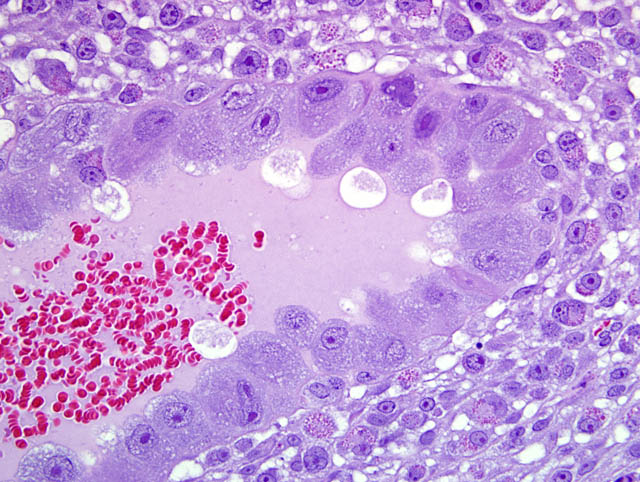
- Giant cell layer: A layer of giant trophoblasts separates maternal-origin tissue (decidua) from embryofetal-origin tissue. Giant trophoblasts are up to 150 um in diameter and have abundant, finely-granular, microvacuolated, pale basophilic cytoplasm that often contains distinct PAS-positive droplets. Nuclei are large and round to oval with finely-stippled or coarsely-clumped chromatin and contain one to ten distinct, variably-sized, irregularly-shaped nucleoli.
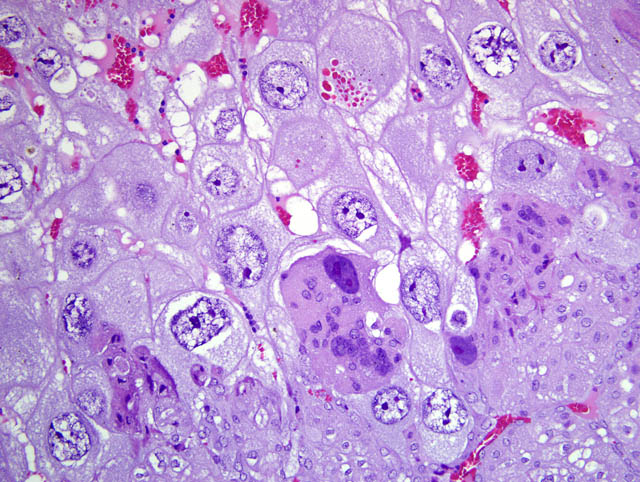
- Trophospongium: Interposed between the giant cell layer and the labyrinth is a band of 20-40 um diameter polygonal cells with moderate amounts of basophilic, vacuolated cytoplasm containing numerous small, PAS-positive granules; these cells are often bi-nucleate with frequent mitoses. Groups of cells comprising this population are separated by fewer large (60-80 um diameter) syncytiotrophoblasts and small vascular lacunae containing mature erythrocytes.
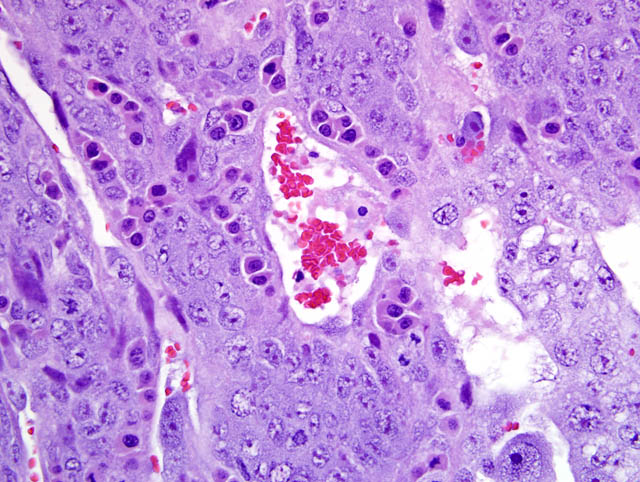
- Labyrinth: The luminal-most layer of the mesometrial side of the uterus is composed of trophoblasts arranged in trabeculae that separate large and small blood-filled lacunae. Trophoblasts contain moderate amounts of basophilic, microvacuolated cytoplasm, and are mitotically active. Larger lacunae contain mature erythrocytes (maternal circulation), while smaller lacunae contain only large, immature, nucleated erythrocytes with pale eosinophilic to basophilic cytoplasm (fetal circulation). Generally, maternal and fetal lacunae are separated by three layers of trophoblasts (hemotrichorial placentation).
In the affected rats of this case the yolk sac placenta is present in the uterine lumen in most histologic sections which consists of a single layer of plump, cuboidal, luminally-oriented cells resting on a 2-5 um thick, glassy, hyaline basement membrane (Reicherts membrane). Employing the morphologic reference data set provided by de Rijk and colleagues (2) as a guide, the placental features described above are interpreted as consistent with a placenta at approximately day 12-14 of pregnancy. However, not all of these features are present on every section, and most slides have two sections, one of which lacks the giant cell layer, trophospongium, labyrinth, and yolk sac altogether. Additionally, other features of a normal rat pregnancy, i.e. a conceptus and fetal umbilical vessels, are conspicuously absent. One possible explanation for the presence of both maternal and embryofetal placental structures in the absence of a conceptus is embryonic death. For example, embryonic death attributed to an intercurrent infection with Sendai virus and Pasteurella pneumotropica resulted in the formation of deciduomas with trophoblastic giant cells in rats (1); admittedly, this unlikely scenario does little to account for the clinical history in this case, and would likely result in significantly more placental pathology than is present in the examined sections.
In light of the lively discussions elicited by this case, the contributor was contacted to confirm the case history and ensure the possibility of pregnancy was excluded. Indeed, the submitted specimens were sampled from several female control rats that were pair-housed in a long-term study, and pregnancy would have been self-evident given the length of the study. Anecdotally, some experienced pathologists have noted extremely well-organized decidual reactions like this in rats that were certainly not pregnant; unfortunately, such examples are not reported in the literature, and we are unable to explain the presence of placental components of apparent fetal origin. A parthenogenetic process was also considered as a possible cause, but deemed exceedingly unlikely in this case given the signalment and clinical history provided by the contributor. Thus, we conclude that the histologic findings are most compatible with pregnancy and very recent embryonic loss. However, these histologic features in a known unmated animal are unique in our experience, and the pathogenesis remains unclear. We extend our sincere thanks to the contributor for submitting this very interesting case, and to the veterinary pathologists who graciously shared their opinions and expertise.
References:
2. de Rijk EPCT, van Esch E, Flik G: Pregnancy dating in the rat: placental morphology and maternal blood parameters. Toxicol Pathol 30:271-282, 2002
3. Elcock LH, Stuart BP, Mueller RE, Hoss HE: Deciduoma, uterus, rat. In: Monographs on Pathology of Laboratory Animals: Genital System, eds. Jones TC, Mohr U, Hunt RD, pp. 140-145. Springer-Verlag, Berlin Heidelberg, 1987
4. Greaves P: Female genital tract. In: Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation, 3rd ed., pp 728-29. Elsevier, London, UK, 2007
5. Leininger JR and Jokinen MP: Oviduct, uterus and vagina. In: Pathology of the Fischer Rat: Reference and Atlas, eds. Boorman GA, Eustis SL, Elwell MR, Montgomery Jr. CA, MacKenzie WF, pp. 450-52. Academic Press, Inc., London, UK, 1990