Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 9
November 1st, 2017
CASE IV: MS17-3649 (JPC 4104252).
Signalment: 3-month-old, male and female, AG129, Mus musculus, mouse.
History: Mice received one foot pad injection of Zika virus. Seven to ten days later, the mice were submitted to necropsy due to weight loss or having been found dead. At presentation, live mice were scruffy, some were unstable on their rear legs and others had developed hind limb paralysis. The animals were in thin - lean body condition with variable hydration status.
Gross Pathology: No gross lesions were present.
Laboratory results: None submitted.
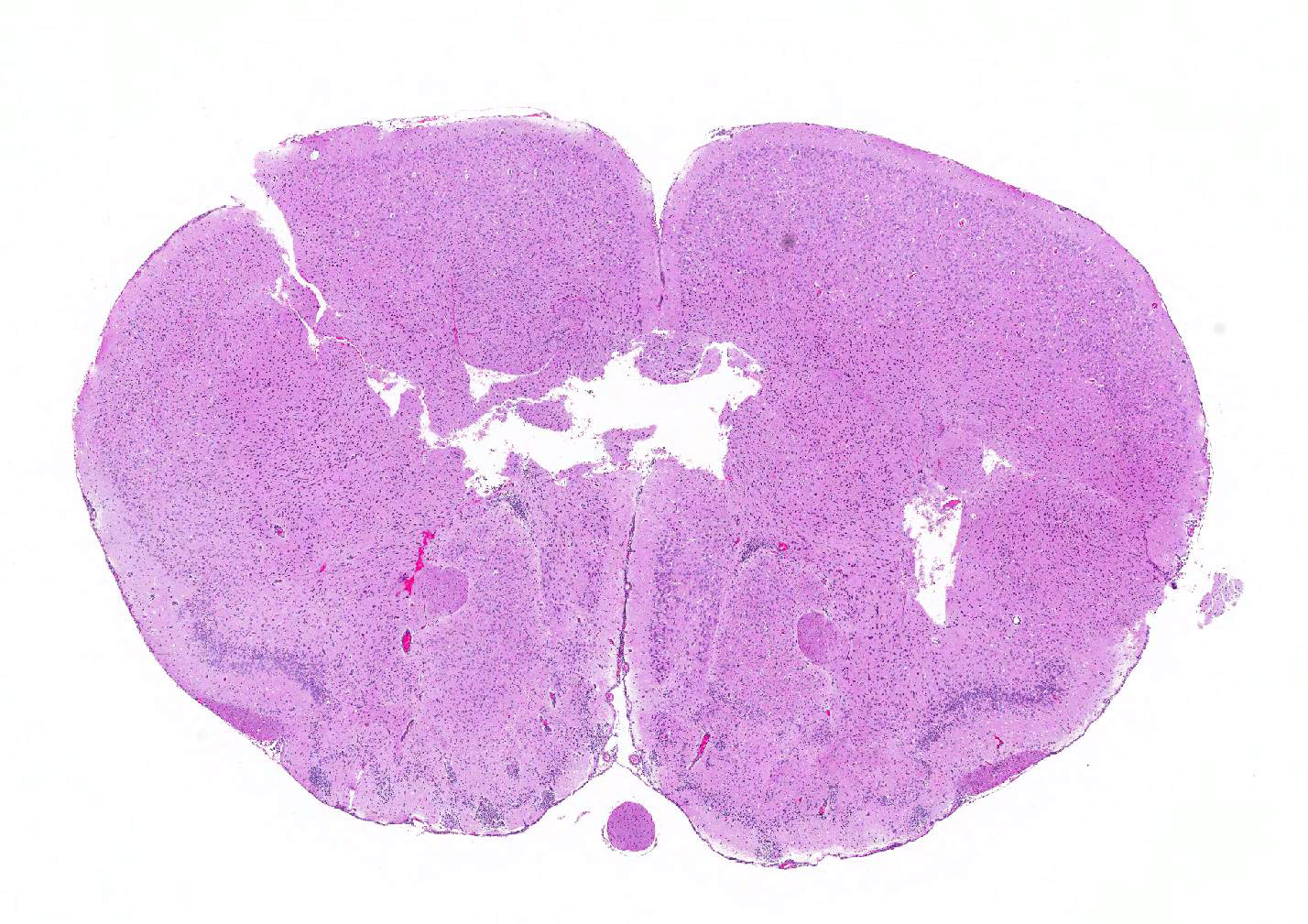
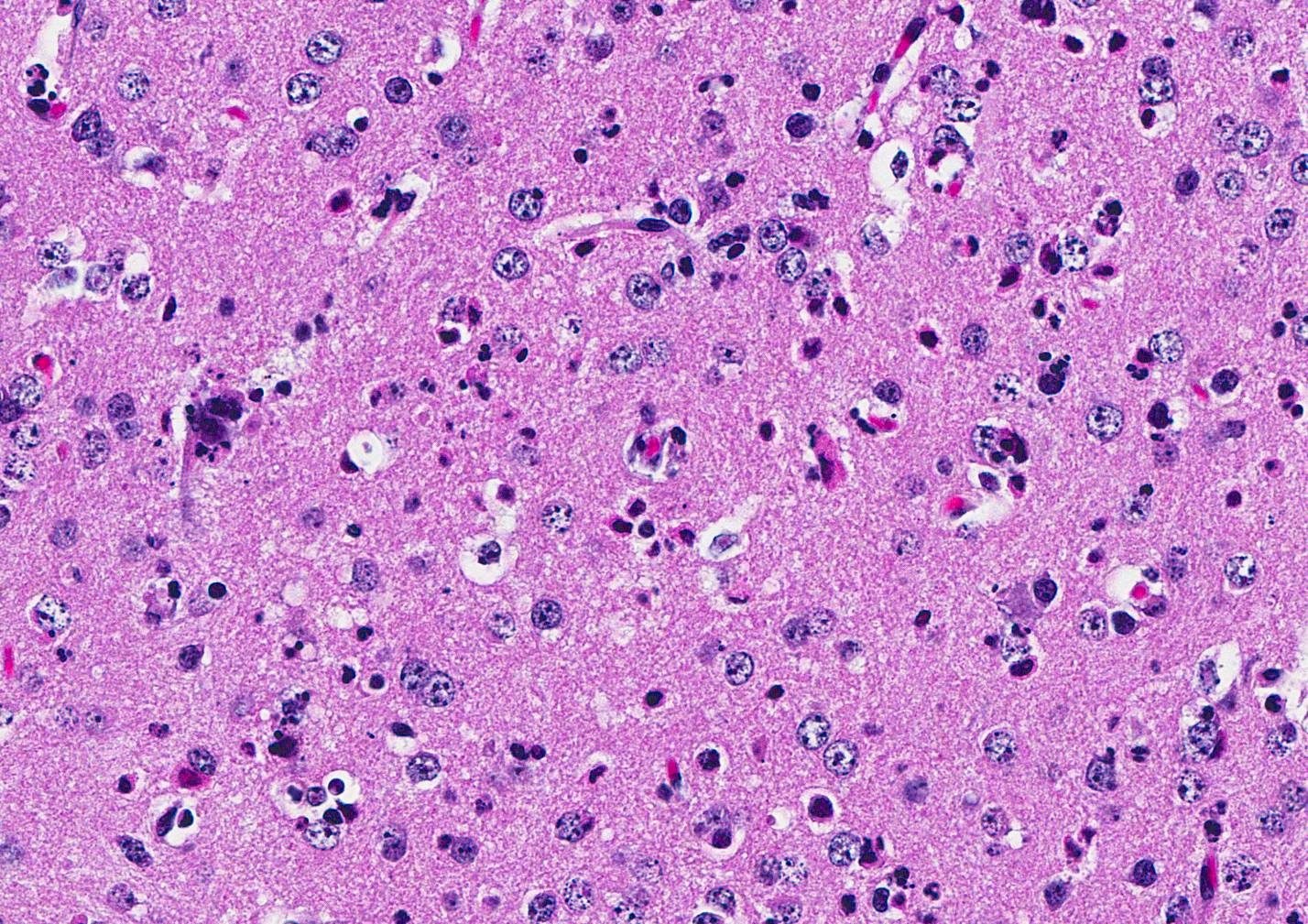
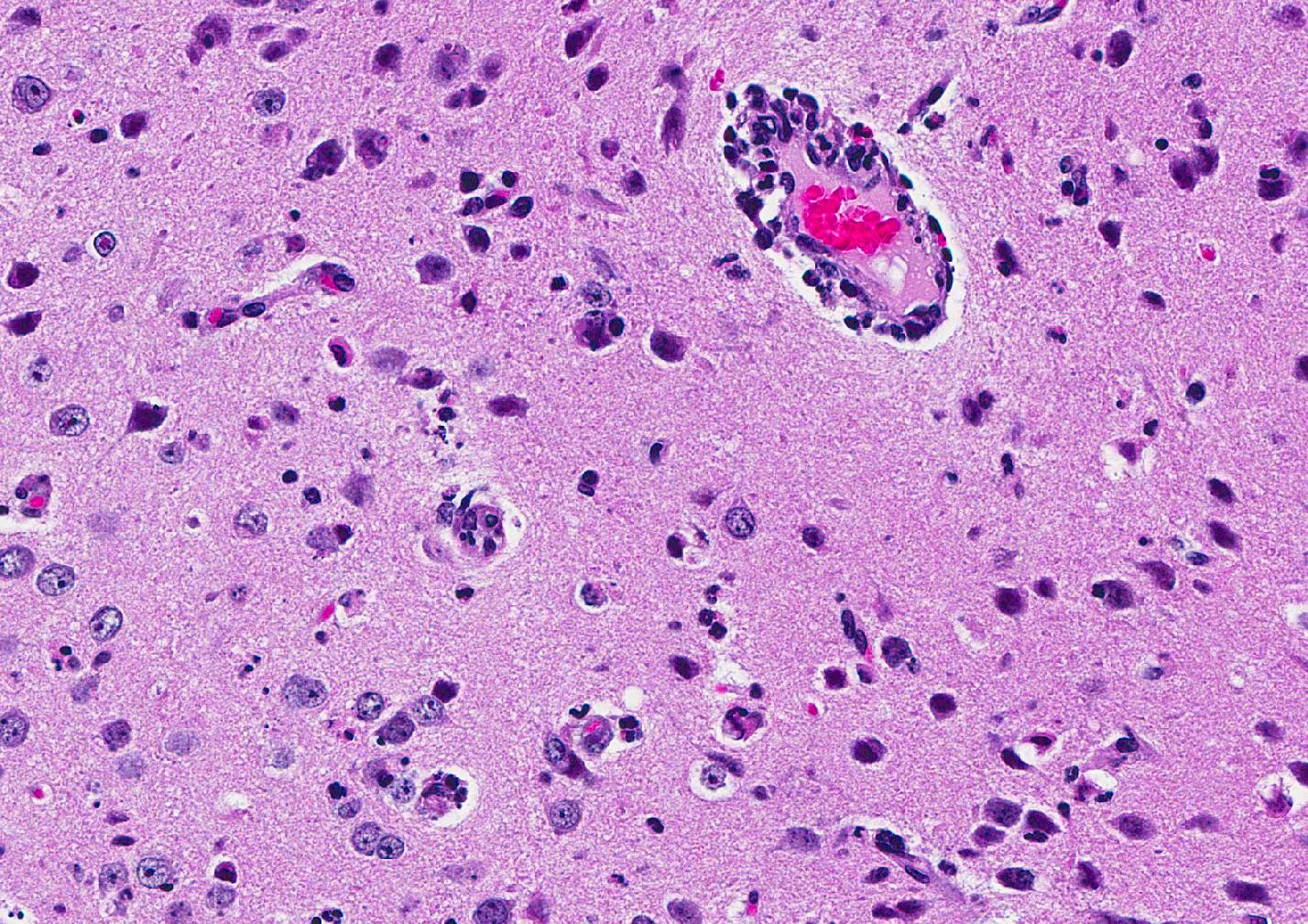
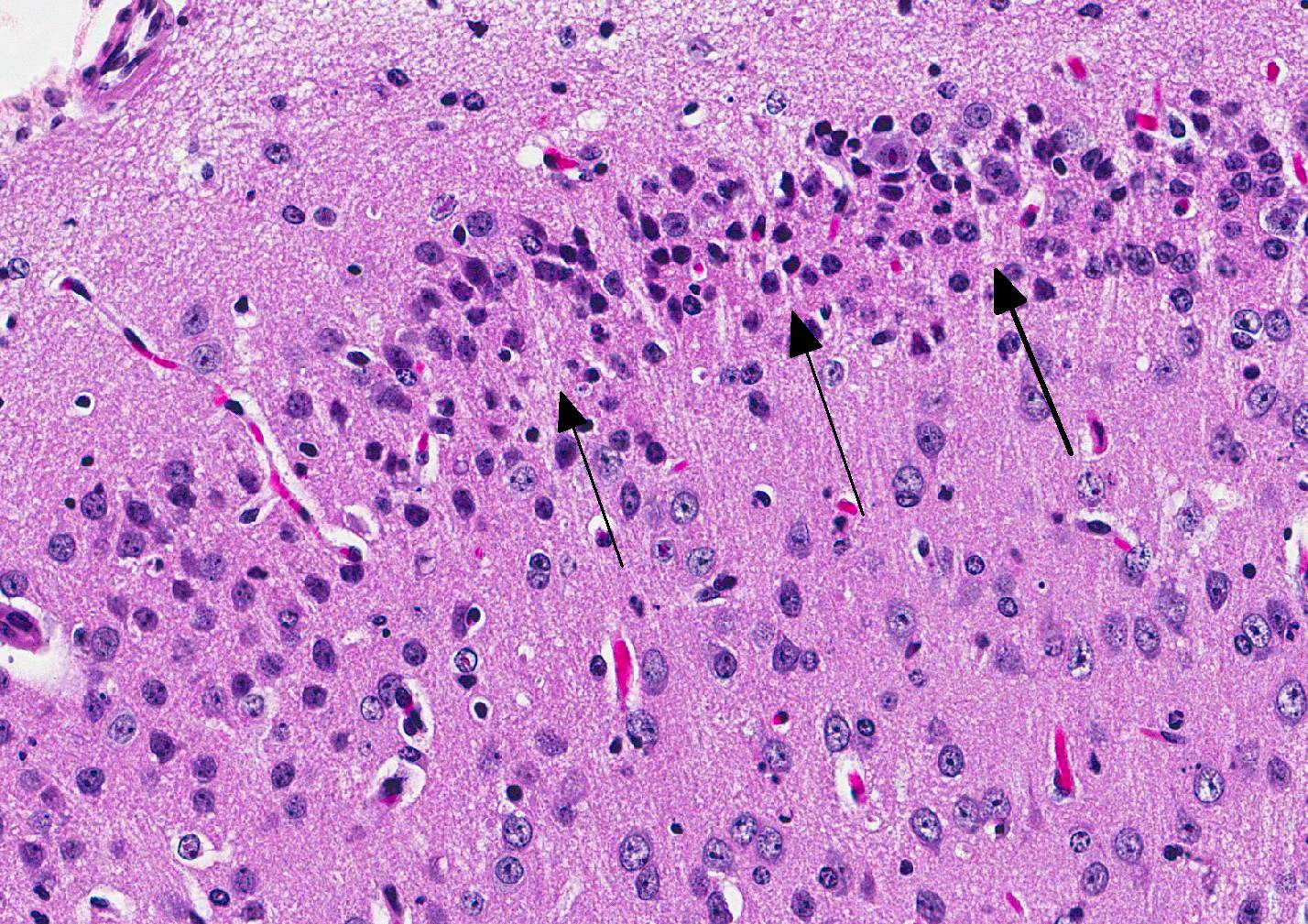
Microscopic Description: Inflammatory changes in the brain were present primarily in the cerebrum and were composed of multifocal, random aggregates of neutrophils, necrotic cells [presumed to be astrocytes] with minimal gliosis. Inflammation varied from minimal - mild and occasionally there were solitary necrotic cells without associated inflammation or interstitial change. Neuronal necrosis varied from none to foci of necrotic neurons. Few vessels had smooth muscle hypertrophy with acute subacute vasculitis. There was variable ependymal cell necrosis. Similar inflammatory and vascular changes were present in the spinal cord. Some sections of cerebellum had mild granular layer necrosis. Purkinje cells appeared to be spared.
There is variation of the severity of the lesions in some slides.
Acute orchitis with seminiferous tubule cell necrosis was the only other microscopic lesion. [Not submitted]
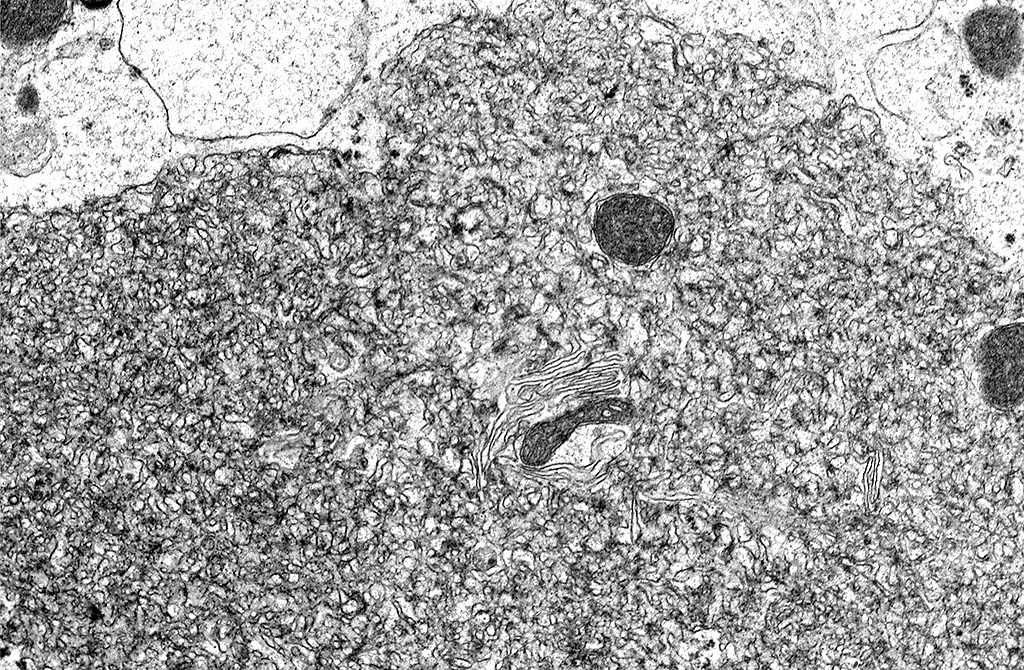
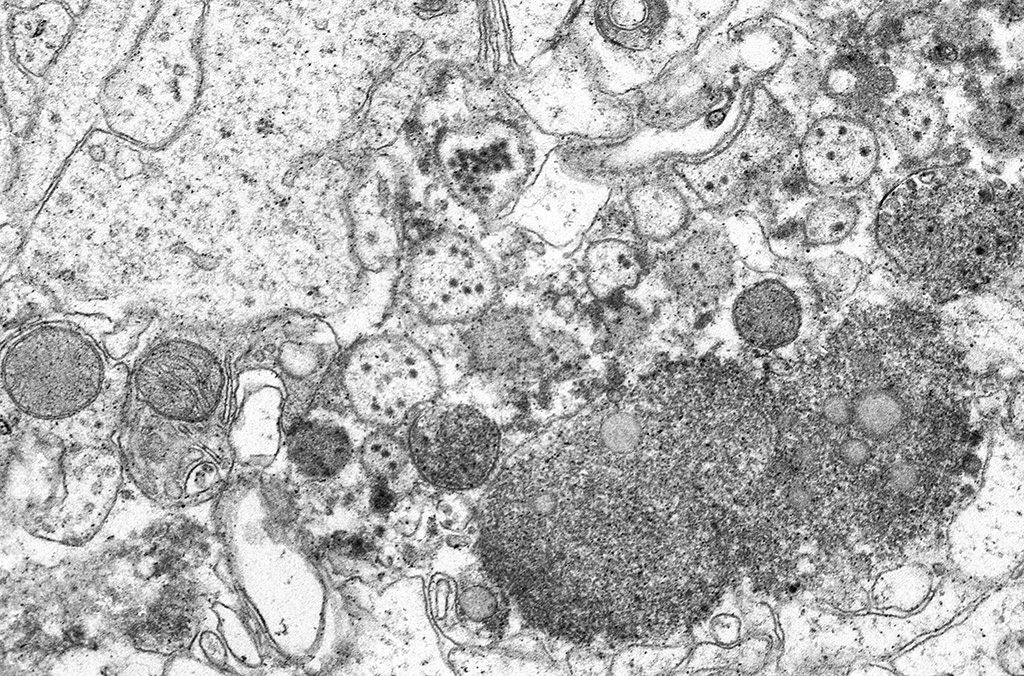
Transmission Electron Microscopy: In the cytoplasm of cerebral axons and dendrites, there were areas with highly expanded and convoluted endoplasmic reticulum (Image #48). Within the endoplasmic reticulum, there were membrane-bound vesicles with numerous unenveloped, 35- 45 nm, round, dark cored particles. Some virions were heavily clustered while others were widely dispersed within the vesicle. There was adjacent cellular debris with a few degenerated mitochondria. (Image #91) The adjacent tissue was unaffected. (Image #55). Bar = 500 nm.
Contributors Morphologic Diagnosis: Meningoencephalitis and myelitis, multifocal, minimal to moderate, subacute with neuronal necrosis and vasculitis.
Contributors Comment: Zika virus is an 11 kb ssRNA Flaviviridae carried by Aedes spp. mosquitoes. Other flaviviruses to which it is related are West Nile, Dengue, and yellow fever. Zika virus infection was first described in 1952 where it was isolated from a febrile sentinel rhesus macaque in the Zika forest of Uganda.3 Since then, the virus has spread to Asia and caused outbreaks in Micronesia in 2007 and French Polynesia in 2013. In 2015, the first case was reported in Brazil.4,7 Since then, there has been widespread infection, 440,000 1.3 million, reported in Central and South America, the Caribbean, and Miami.2,5,8 The Ministry of Health of Brazil has reported there has been a 20-fold increase in microcephaly.6
Infection via Aedes spp. mosquitoes is the most common route of infection. Viral replication occurs within mosquitos which, when taking a blood meal, then infect humans. Nonhuman primates and other mammals may serve as reservoir hosts. Aedes range is global but most species are concentrated in tropical and subtropical areas. The virus can also be transmitted as a congenital/perinatal or sexual infection. 6,7,8 Transmission via blood transfusion also has been reported.7
It is thought that after a mosquito bite, the virus enters through fibroblasts, keratinocytes and immature dendritic cells, migrates to lymph nodes and blood.8 Once in the cell, the virus induces marked proliferation of the endoplasmic reticulum, rearrangement of the ER membranes, and formation of vesicles, typical of flaviruses. The virus then replicates within the vesicles to form immature virions. The vesicle passes into the Golgi apparatus, becomes glycosylated in and then, being released from it, undergoes final maturation in cytoplasmic vesicles and is released as a mature virion.7
Approximately 80% of Zika virus infections are asymptomatic. Infection may be associated with fever, rash, arthralgia, myalgia, headache and conjunctivitis and signs tend to resolve in 2 weeks.4,6,7 Similar symptoms can also be seen Dengue and Chikungunya virus infection. Individuals may go on to develop meningitis and meningoencephalitis. Additional reported sequelae include hearing loss, hematospermia, hypotension and genitourinary symptoms. Infection in Asia and the Americas has been associated with an increase in Guillain-Barré syndrome. Death is not common but has been reported in debilitated/immune-suppressed individuals.2,8
Tests to detect Zika can be performed on: CSF, blood, serum, amniotic fluid, fetal and placental tissues, urine, and saliva. During the acute phase of infection, RT-PCR is the best test in which to confirm for Zika virus. However, the period that RNA is present may be as short as 5 days. Serology may also be used but results may be confounded by previous flaviviral infection and time between infection and testing.8
Autopsy findings in a small cohort of ten term neonates included low brain weight, microcephaly with thinning of cerebral cortices with overlapping sutures, pachygyria or agyria and increased ventricle size; thin corpus callosum and optic chiasm; thickening of the leptomeninges; and calcifications between grey and white matter. Other findings included aqueduct stenosis, cerebellar hypoplasia, brainstem abnormalities, and thin, distorted spinal cords. All the neonates had arthrogryposis and some were microphthalmic.2 Infectious agents that are known to cause similar lesions in infants: intrauterine growth restriction, microcephaly and calcifications, conjunctivitis, hearing loss, rash, hepatosplenomegaly, or thrombocytopenia, are known by the acronym TORCH: Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19, HIV), Rubella, Cytomegalovirus (CMV), and Herpes.
Microscopic lesions appeared to be secondary to abnormal migration and cerebral neuronal/glial loss. Neurons were seen in abnormal locations, as immature cell aggregates around the ventricles, with astrocytosis and calcification. Myelin fibers were decreased or absent. Axonal changes included degeneration, spheroid formation, and loss of orientation. Branching tubes replaced the normal aqueduct. The spinal cord was small due to small cortico-spinal tracts with nerve cell degeneration, loss, gliosis and calcification. Dorsal root ganglia were preserved but ventral ganglia were not. Inflammation was not a prominent feature and when seen, was composed of lymphocytic meningitis, perivascular cuffing and histiocytes and gliosis. Electron microscopy revealed numerous intravesicular virions. Muscle atrophy, mild hepatitis and pituitary, retinal and lens mineralization rarely were seen.
Placentas were small for gestational age with inflammation of the villi, fetal membranes and decidua, stromal fibrosis, smooth muscle hyperplasia of villous vessels, chorionic vasculitis, increased vascularity and stromal calcification. In this study, maternal infection in the first or early second trimester led to more severe congenital anomalies.2
Until recently, mouse models of Zika have been few. Wild type B6, CD-1, BALB/C mice tend be resistant to Zika infection. However, they can be made to be susceptible by treatment with steroids or mAbs directed against interferon ?/? receptors.5 AG129 mice lack interferon ?/? and ? receptors and A129 mice (lacking interferon ?/? receptor) are susceptible to Zika infection. As seen in our mice, AG129 mice develop neurologic signs, weight loss and subsequently die but have no gross lesions. Microscopic lesions were limited to the CNS1. In some studies, the testis is also affected.5 The mice have high viral loads are found in brain, spinal cord, spleen and testes and may be studied to determine the pathogenesis of viral dissemination.5
JPC Diagnosis: Brain, multiple levels: Meningoencephalitis, necrotizing and neutrophilic, diffuse, moderate with marked neuronal and glial necrosis, AG129 (Mus musculus), mouse.
Conference Comment: The conference moderator, who also submitted the case, reviewed the key clinical, gross, and microscopic features of Zika virus (see contributors comment above). Conference attendees noted necrotic cells randomly arranged throughout the submitted samples (no spinal cord was included) and aggregates of viable and degenerate neutrophils and necrotic cellular debris. Cells are so shrunken it is difficult to determine cell type but most attendees suspect the majority of necrotic cells are glial cells. The conference moderator showed images from additional tissues including spinal cord and testis. Both of which had a remarkable infiltration of neutrophils and associated necrosis. Within the testis the interstitial space is expanded up to 4 times by neutrophilic inflammation and the spinal cord has degenerative neurons, neuronal necrosis, and spongiosis predominately within the grey matter. There are also numerous astrocytes and neurons that contain eosinophilic intranuclear inclusions, attendees assumed they were viral inclusions. Transmission electron microscopy (TEM) images were provided that clearly identified virions within the cytoplasm but not within the nucleus of affected cells. The pink material seen microscopically cannot be accounted for yet and will most likely be the subject of continued investigation.
Veterinary Pathology
Division of Veterinary Resources
Office of Research Services
National Institutes of Health
https://www.ors.od.nih.gov/sr/dvr/drs/Pages/default.aspx
References:
1. Aliota MT, Caine EA, Walker EC, Larkin KE, Camacho E, Osorio JE. Characterization of lethal Zika virus infection in AG129 mice. PLoS Negl Trop Dis. 2016; 10(4):19.
2. Chimelli L, Melo ASO, Avvad-Portari E, Wiley CA, et al. The spectrum of neuropathological changes associated with congenital Zika virus infection. Acta Neuropathol. 2017;133(6):983-999.
3. Dick, GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46(5):521-34.
4. Driggers RW, Ho CY, Korhonen EM, Kuivanen S, et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med. 2016;374(22):2142-2151.
5. Morrison, TE, Diamond, MS. Animal models of Zika virus infection, pathogenesis, and immunity. J Virol. 2017;91(8):29.
6. Mlakar J, Korva M, Tul N, Popovi? M, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951-958.
7. Offerdahl DK, Dorward DW, Hansen BT, Bloom ME. Cytoarchitecture of Zika virus infection in human neuroblastoma and Aedes albopictus cell lines. Virology. 2017;501:54-62.
8. Plourde AR, Bloch EM. A literature review of Zika virus. Emerg Infect Dis. 2016;22(7):1185-1192.