Signalment:
Cat (
Felis silvers cats), 12-year-old spayed female mix breed.A 12-year-old mix breed cat was referred with a two-month history of persistent vomiting and loss of
weight. At palpation an abdominal mass was detected. Ultrasound showed a heterogeneous and thickened gastric
wall. Laparotomy revealed an intramural ulcerated mass close to the pyloric sphincter that was completely removed.
The cat was still alive 5 months after surgical resection.
Gross Description:
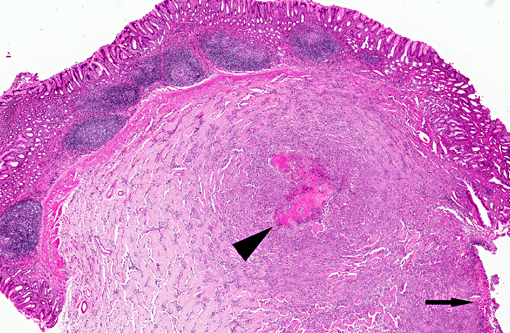
Gross examination of surgical biopsy showed a severely ulcerated mucosa with a diffuse and
severe thickening of the gastric wall that appeared grayish.
Histopathologic Description:
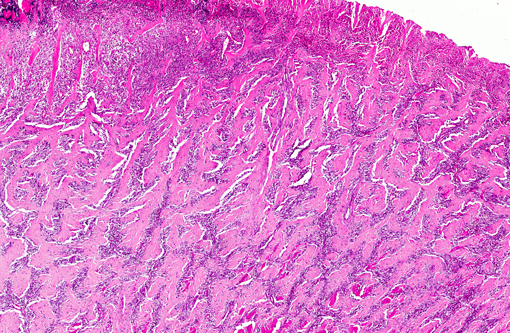
The histological appearance of the gastric lesion consisted of an
ulcerated and fibrinous exudative mass expanding and replacing the wall at the pyloric/fundic junction. The lesion
affected the inner layers (
mucosa and
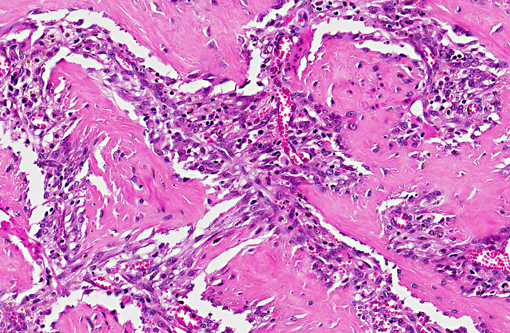
tunica muscular is) of the gastric wall and consisted of branching and
anastomosing trabeculae of dense collagen separated by a densely cellular population of spindle-shaped cells
(sclerosing fibroplasia). The trabecular collagen merged into typical granulation tissue at the periphery of the lesion.
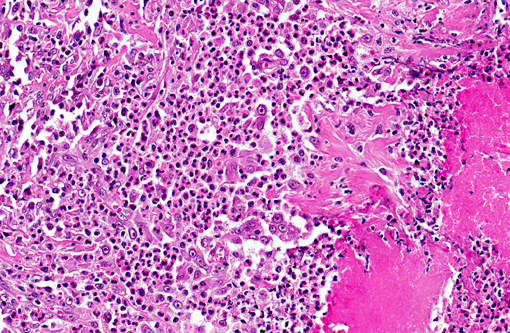
The fibroplasia contained variably dense infiltrates of eosinophils, mast cells, and fewer neutrophils, lymphocytes,
and plasma cells. Multifocally there were necrotic foci with intralesional bacterial colonies and prominent
eosinophils.
Morphologic Diagnosis:
Stomach: gastric eosinophilic sclerosing fibroplasia.
Condition:
Gastric eosinophilic sclerosing fibroplasia
Contributor Comment:
We report a lesion in which eosinophilic inflammation is the predominant feature and
which appears to be limited to the gastrointestinal tract. The presentation is typical of a feline gastrointestinal
eosinophilic sclerosing fibroplasia as previously described(1). Grossly, the lesion is an ulcerated mass at the pyloric
sphincter or ileocecocolic junction which, due to the presence of dense sclerotic fibroplasias, can be clinically
misdiagnosed as a neoplasm. Microscopically, , the characteristic trabecular and anastomosing pattern of dense
collagen which resembles osteoid, and the presence of numerous mast cells may lead to the incorrect diagnoses of
osteosarcoma or sclerosing mast cell tumor. In the present case, bacteria were detected histologically at the center of
necrotic foci enclosed in the submucosa. In a previous study of 25 cats(1) with similar eosinophilic sclerosing lesions,
15 had intralesional bacteria; Gram-negative rods were present in most of the lesions. On the contrary in Ozakis
et
al study(6), gram-positive cocci (specifically, methicillin-resistant
Staphylococcus aureus) were the most frequent.
The significance is unknown. One hypothesis is to consider that bacterial organisms could initiate these lesions.
However the pyloric sphincter and ileocecocolic junction locations suggest that bacterial action could be combined
with physical forces, such as mucosal foreign-body penetration. The reasons for the fibroplasia and eosinophilic
response are not clear. Activated eosinophils can produce numerous mediators such as TGF-+�-� and IL-1 that can play
a role in fibrosis by increasing extracellular matrix and fibroblast proliferation. The prominent eosinophilic
infiltration in cats occurs as part of the feline eosinophilic granuloma complex or in response to a variety of stimuli,
including parasites, viruses, bacteria, and fungi.Â
Toxoplasma gondii was reported to cause eosinophilic fibrosing
gastritis in cats(5). An inherited eosinophilic dysregulation was also suspected in cats with eosinophilic granuloma
complex, and in a recent study(1), hypereosinophilia was reported in 58% of tested cats, suggesting that genetically
predisposed cats could develop eosinophilic inflammation in response to the penetration of bacteria into the gastrointestinal
wall secondary to mucosal foreign-body penetration.
JPC Diagnosis:
Stomach: Necrosis, focally extensive, with marked fibrosis and eosinophilic and histiocytic
inflammation.
Conference Comment:
The etiopathogenesis of feline gastrointestinal eosinophilic sclerosing fibroplasia (ESF)
remains controversial, with various hypotheses including sclerosing mast cell tumor (sMCT) and abcess-forming
inflammatory granulation tissue with eosinophilic infiltration. It is also possible that ESF represents the common
end point of any of a number of neoplastic or inflammatory processes; thus it is important to be aware of the
potential differential diagnosis and diagnostic dilemmas presented by ESF(1, 2, 3, 4, 7).
Conference participants agree with the contributor in this case and favor the diagnosis of feline gastrointestinal
eosinophilic sclerosing fibroplasias (ESF), likely due to some previous inflammatory condition in the
gastrointestinal tract. There is some significant slide variation, and some sections may not contain identifiable
pylorus.
sMCT and ESF have several areas of major difference, including location, clinical pathology, clinical findings,
survival time, and the presence of bacteria within the lesions. In sMCT, 76% of the lesions were located in the small
intestine compared to only 16% for ESF. The majority of ESF cases are located mainly at the pyloric sphincter of
the stomach as in this case (48%) or the ileocecocolic junction (24%). 58% of ESF had a peripheral eosinophilia,
which was not reported in sMCT, and 100% of ESF had a palpable mass, compared to only 17% with sMCT. While
both conditions showed spread to local lymph nodes, this only occurred in 28% of ESF cases as compared to 66%
with sMCT, and there was metastasis to the liver in 66% of cases of sMCT and no report of hepatic spread with
ESF. Grossly, ESF presented as an ulcerated mass expanding the wall of the stomach or intestine and
microabscesses or necrotic foci with cultured bacteria were present in 56% of cases and in all ileocecocolic and
colonic cases, while sMCT presented as a marked transmural expansion of the small intestine by a firm,
homogenous tan mass with abrupt mucosal ulceration in 58% of cases with no intralesional bacterial colonies and
occasional surface colonies in ulcerated areas. The survival time with sMCT was much lower with an average of
92% of patients dead within 2 months of diagnosis compared to a variable survival time with ESF, depending on
different circumstances, such as intestinal perforation or euthanasia, although cats treated with prednisone had a
significantly higher survival time of up to 2 years(1, 2, 3, 4, 7).
There are areas of similarity with these two conditions which gives rise to much of the controversy with these
entities. In both conditions, there is a marked trabecular pattern of dense collagen, a heavy infiltrate of eosinophils,
and the presence of mast cells (either the neoplastic component or an infiltrate of physiologic mast cells). Also, there
is spread to local lymph nodes in both cases, which present as diffuse effacement of the lymph node architecture by
dense collagenous trabeculae and multifocal microabscesses with ESF, and replacement of nodal architecture by
neoplastic mast cells in sMCT. Both conditions have a fairly low mitotic rate, with 1 per 20 400x high power fields
with sMCT and a variable number in ESF. Clinical signs were similar between each condition, which is expected,
including similar rates of presentation for vomiting and weight loss(1, 2 ,3, 4, 7).
References:
1.Craig LE, Hardam EE, Hertzke DM, Flatland B, Rohrbach BW, Moore RR. Feline gastrointestinal eosinophilic sclerosing fibroplasia. Vet Pathol
1: 63-70, 2009.
2. Gamble DA. Letter to the editor #2.Â
Vet Comp Oncol 2010; 8:235-6.
3. Hasley CHC, Powers BE, Kamstock DA. Feline intestinal sclerosing mast cell tumour: 50 cases (1997-2008).Â
Vet Comp Oncol. 2010; 8:72-79.
4. Kamstock D, et al. Authors response.Â
Vet Comp Oncol 2010; 8:236-242.
5. McConnell JF, Sparkes AH, Blunden AS, Neath PJ, Sansom J. Eosinophilic fibrosing gastritis and toxoplasmosis in a cat. J Feline Med Surg
9 : 82-87, 2007.
6. Ozaki K, Yamagami T, Nomura K, Haritani M, Tsutsumi Y, Narama I. Abscess-forming inflammatory granulation tissue with Gram-positive cocci and prominent eosinophil infiltration in cats: possible infection of
methicillin-resistant Staphylococcus.Vet Pathol
40:283-7, 2003.
7. Schulman FY, Lipscomb TP. Letter to the editor.
Vet Comp Oncol 2010; 8:234-5.