Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 16
January 24th, 2018
CASE II: 1704 0830 (JPC 4101141).
Signalment: 1-year-old, female, Suri alpaca, Vicugna pacos, alpaca.
History: The alpaca was recently found depressed with decreased appetite. She had free access to a small Bermuda grass pasture but no known exposure to any toxic plants. She had no history of any medical treatment. Upon presentation, she was weak, dyspneic, recumbent and unable to stand. Physical examination showed dilated pupils with absence of pupillary light reflexes. Euthanasia followed by necropsy examination was elected by the owner with the aim of identifying any potential herd health problems.
Gross Pathology: The wall of the ileum was mildly and subjectively thickened, but the mucosa was grossly unremarkable. In multiple segments of the spiral colon, the mucosa was uniformly reddened and mildly thickened with multifocal dark red, ulcerative foci overlain by a moderate amount of fibrin. The liver was diffusely pale with an accentuated lobular pattern and greasy texture on cut surfaces (hepatic lipidosis) with multiple petechial and occasional ecchymotic hemorrhages scattered throughout the hepatic parenchyma. In the heart, multifocal petechial hemorrhages were present in the endocardium and myocardium of the left ventricle.
Laboratory results: An iStat Chem 8 biochemical analysis performed upon presentation revealed mild acidemia (AnGap 36 mEq/L; reference interval: 14-21 mEq/L), mild hypoglycemia (89 mg/dL; reference interval: 102-149 mg/dL) and azotemia (creatinine of 6.3 mg/dL; reference interval: 1-2.4 mg/dL).
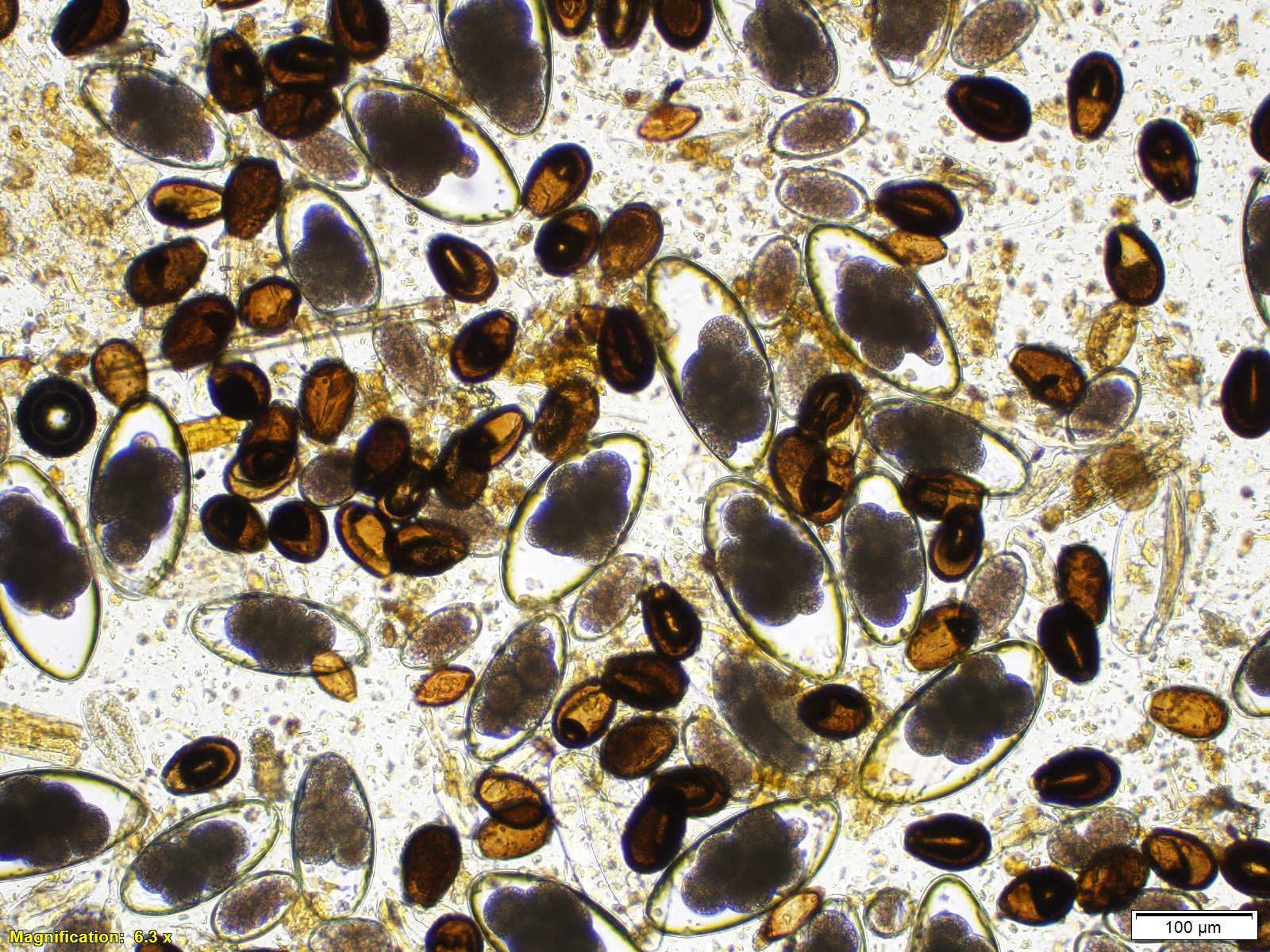
Fecal flotation revealed numerous Eimeria macusaniensis oocysts, Nematodirus sp. eggs, and moderate numbers of Trichostrongyle-type of eggs, Capillaria sp. eggs and Trichuris sp. eggs. Eimeria macusaniensis oocysts were also observed in the direct fecal smears.
Aerobic bacterial culture recovered very large numbers of Escherichia coli and the anaerobic culture recovered very large numbers of Clostridium perfringens from the ulcerated spiral colon. The bacterial culture for Salmonella spp. was negative.
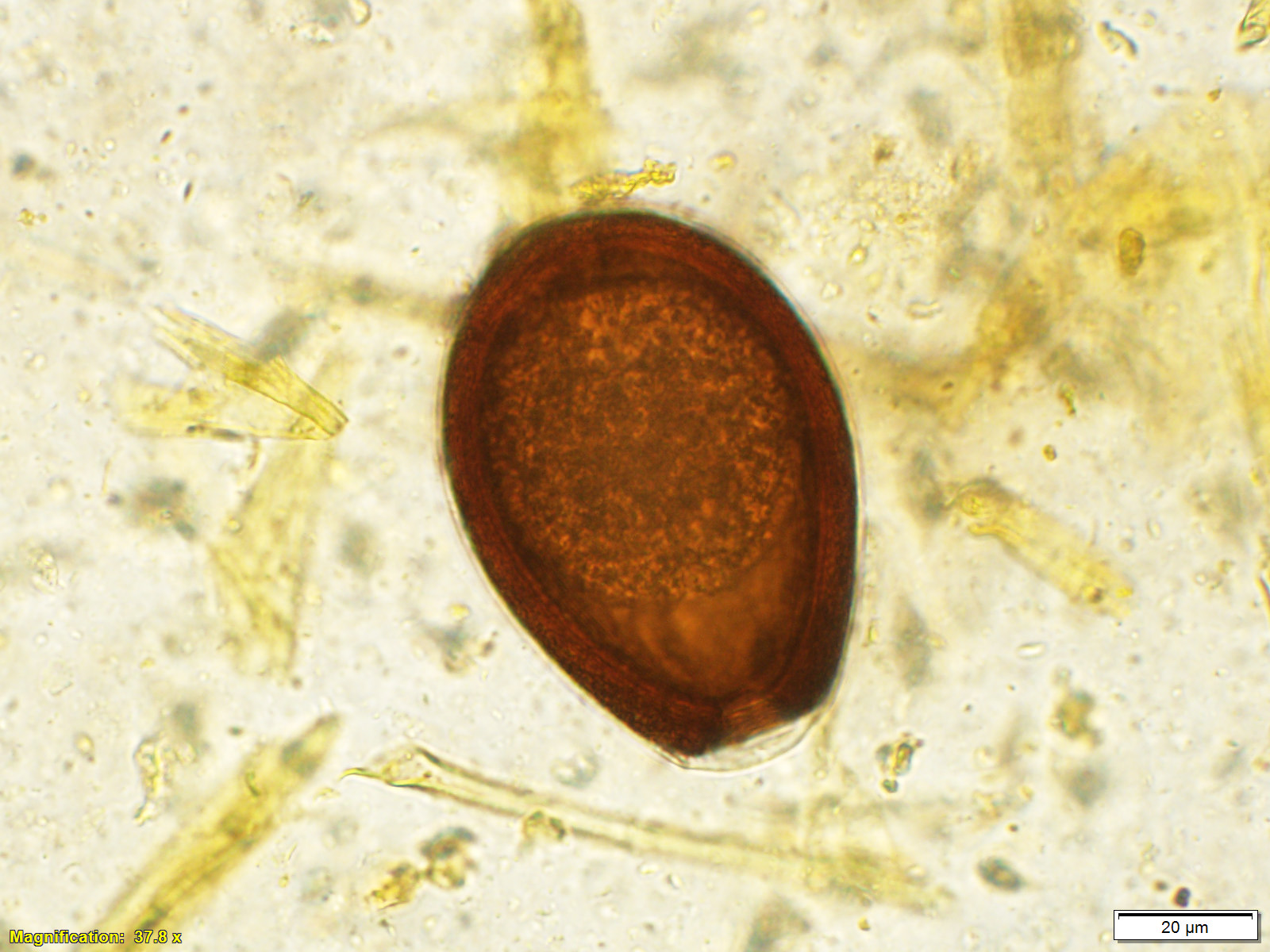
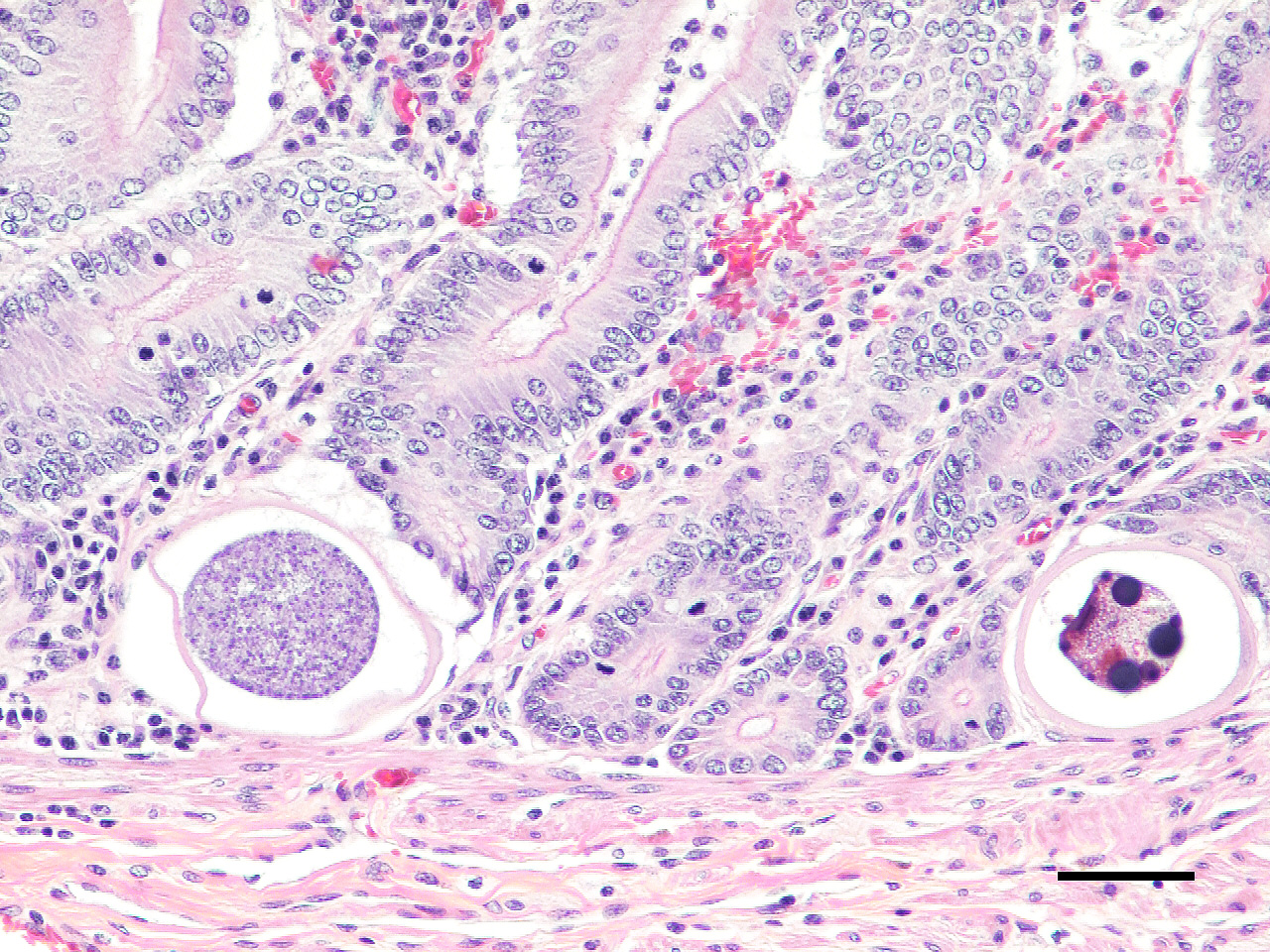
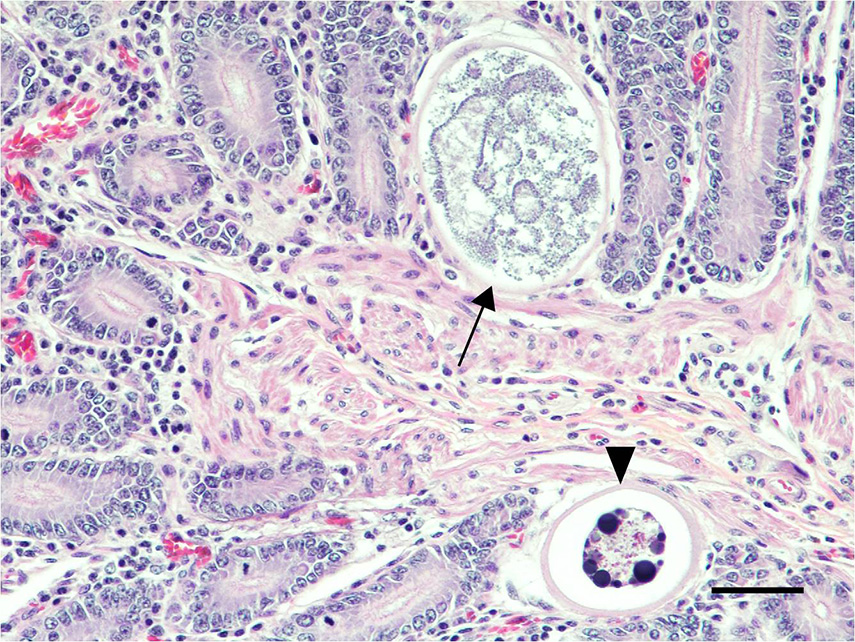
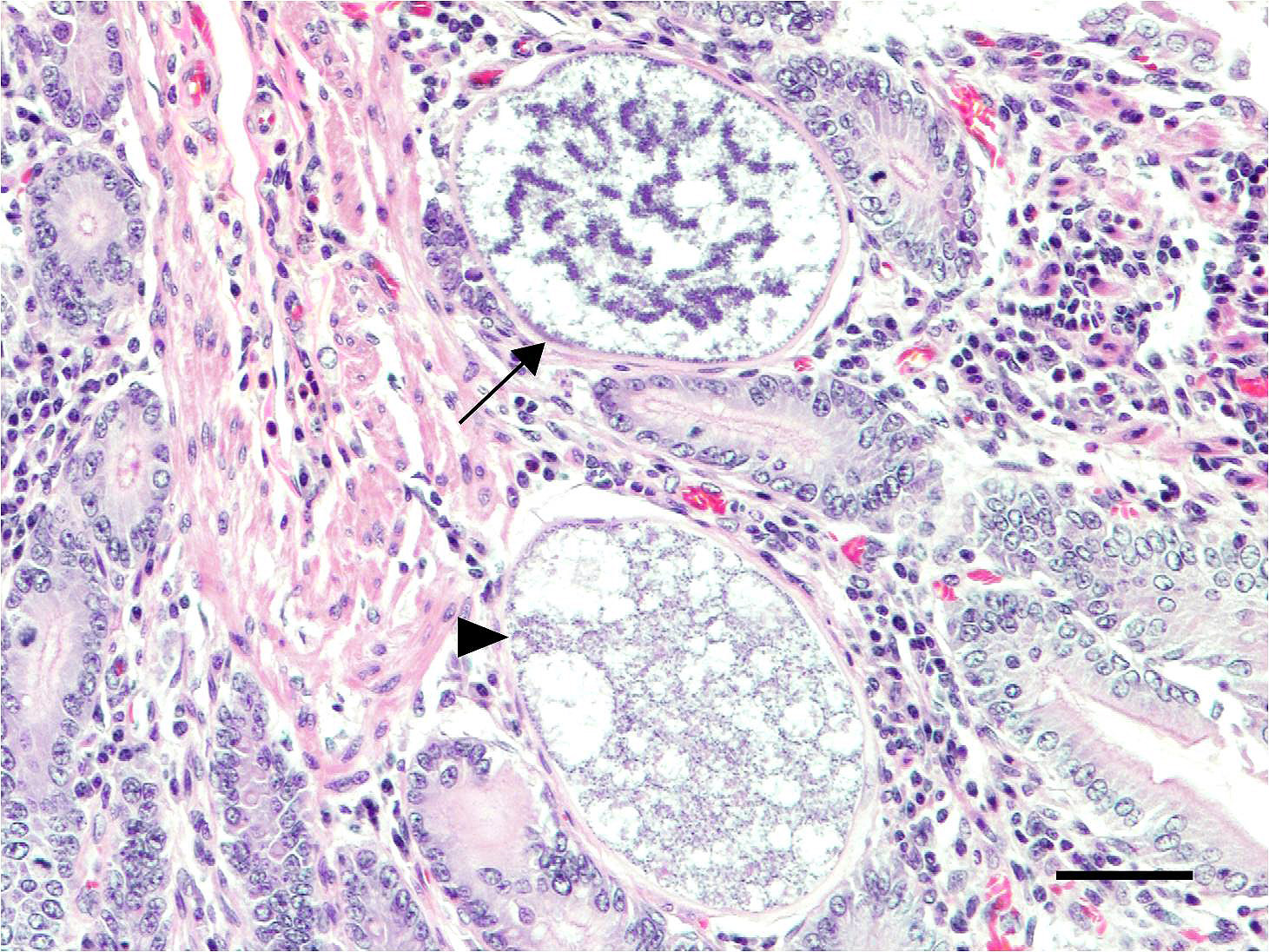
Microscopic Description: Small intestine: The lamina propria is moderately expanded by inflammatory infiltrates, predominantly lymphocytes, plasma cells and eosinophils, as well as large numbers of intra-epithelial, intracytoplasmic protozoan schizonts, gamonts, and oocysts. Most of these protozoa are located in the deep mucosa. The protozoa are present in the cytoplasm of cryptal epithelial cells, compressing the nuclei of the host cells. Schizonts are 70-100 µm in diameter, round to elliptical, and are surrounded by a large, clear parasitophorous vacuole. Within schizonts, numerous small, basophilic merozoites and occasional blastophores are discernible. The microgamonts are 120-200 µm in diameter and contain multiple highly condensed nuclei arranged in irregular bands, anastomosing trabeculae or circular patterns with prominent blastophores formation. Fully-developed microgamonts contain numerous linear or needle-shaped microgametes. In comparison, the macrogamonts are relatively small (50-90 µm in diameter) and are surrounded by a prominent parasitophorous vacuole. The developing macrogametes exhibit multiple, large, variably-sized, characteristic wall-forming bodies at the periphery of the cytoplasm. A few non-sporulated oocysts, approximately 80 x 55 µm, are seen in the epithelial cells or free in the intestinal lumen. The oocysts are pyriform with thick (8-10 µm) walls and have a visible micropyle and micropylar cap. The mucosa associated lymphoid tissues (Peyer’s patches) are prominent and hypercellular.
Spiral colon: Multiple mucosal erosions are accompanied by acute necrosis of surface epithelial cells characterized by shrunken, pyknotic nuclei and hypereosinophilic cytoplasm. The erosive foci are overlain by karyorrhectic cellular debris intermingled with degenerate neutrophils, fibrin, mucus, erythrocytes and bacterial colonies. There are mildly increased numbers of lymphocytes, plasma cells and eosinophils present in the lamina propria of the affected segments.
Contributor’s Morphologic Diagnosis:
Ileum: Moderate, subacute, diffuse lymphocytic and eosinophilic ileitis with numerous intralesional protozoan schizonts, gamonts and oocysts (morphologically consistent with Eimeria macusaniensis).
Spiral colon: Moderate, acute, multifocal erosive colitis, with intralesional bacterial colonies.
Contributor’s Comment: This is a presumed case of acute sepsis secondary to erosive colitis with coexistence of intestinal Eimeria macusaniensis. E. macusaniensis has been recognized as a significant intestinal pathogen in alpacas and llamas.1,2,4,5,6 Along with the other 5 species of Eimeria known to infect camelids (E. lamae, E. alpacae, E. punoensis, E. peruviana, and E. ivitaensis), coccidiosis in alpacas was generally considered subclinical and only occasionally caused disease in young animals.5 However, recent reports suggest E. macusaniensis infection in both adults and juveniles can cause varying degrees of clinical disease and death, either by sole infection, co-infection with other Eimeria spp., or complicated by secondary bacterial infections.2-4 It appears E. macusaniensis infected animals may be predisposed to other diseases.1 A relationship between enterotoxemia caused by Clostridium perfringens and E. macusaniensis infection in alpacas has been proposed and described.4,6 In the present case, the erosive colitis, potentially caused by C. perfringens overgrowth, may have been predisposed by the heavy burden of E. macusaniensis.
Although the small intestine was grossly unremarkable except for subjective, mild thickening of mucosa of the ileum, large numbers of asexual and sexual stages of E. macusaniensis were revealed histologically. E. macusaniensis is recognized by the distinct, relatively large oocysts with average sizes of 75.5 μm by 54.9 μm during endogenous stages in tissue sections7 and 106.6 μm by 80.5 μm in the feces.6 The oocysts are pyriform shape, with brown, 9-10 μm thick walls, a micropyle and a micropylar cap.7,8 Other endogenous stages of E. macusaniensis (schizont, macrogamont and microgamont) are reported to be relatively indistinguishable from other Eimeria spp. in camelids.5,7
Antemortem diagnosis of E. macusaniensis can be challenging due to several factors, including nonspecific clinical signs, relatively long pre-patent period (?30 days), and variable numbers of oocysts shed in the feces of affected animals. In addition, the oocysts of E. macusaniensis have a relatively high specific gravity, which makes it possible for them to be missed by routine fecal flotation methods and requires specific techniques (e.g. saturated sugar solution or flotation solutions with higher specific gravity) to achieve a better detection rate.2,4 Therefore, the prevalence of E. macusaniensis may sometimes be underestimated.
In a report of 34 alpacas and 15 llamas,2 the most commonly reported clinical signs of animals infected by E. macusaniensis include lethargy, anorexia and weakness with few cases presented with diarrhea and colic. It is also important to note that there is no significant age or sex predisposition, although juveniles and females at breeding ages comprise a considerable subset among the infected population, which is hypothesized to be associated with the depressed immune status of the animals.2
JPC Diagnosis:
- Ileum: Intraepithelial apicomplexan schizonts, gamonts, and oocysts with minimal lymphocytic inflammation, Suri alpaca (Vicugna pacos), alpaca.
- Spiral colon: No abnormal findings.
Conference Comment: There are innumerable species of the genus Eimeria which demonstrats a direct coccidian life cycle characterized by both sexual and asexual reproduction. The Eimerian life history is prototypical and may be used as a basis for understanding the life cycle of all other coccidians. Rare exceptions include coccidians that utilize blood-feeding arthropods as hosts (Hepatozoon or Schllackia) where the oocysts develop in the arthropod and the mammalian host is infected by ingesting the infected arthropod.1
The life cycle begins when an infective sporulated oocyst is ingested by an appropriate host. There the sporozoites emerge, and schizogony, or asexual replication, begins. Free sporozoites enter cells of the lamina propria or enterocytes within the duodenum, form round trophozoites, and divide to become first-generation schizonts (same as meronts). It is worth noting that all intracellular forms of Eimeria (trophozoite, schizont, etc.) are all located within a parasitophorous vacuole which is best seen on electron microscopy. The first-generation schizont subsequently forms first-generation merozoites that burst out of the infected cell and infect adjacent cells to become second-generation schizonts. Schizogony continues in a cyclical fashion for two to three generations and then final merozoites (telomerozoites) infect a final naïve host cell and develop into either a female macrogamont or male microgamont. At this point sexual replication, or gametogony, can occur. When gamonts mature, they are called gametes. The female macrogamete is unicellular and fills the entire parasitized cell, whereas the male microgamont undergoes multiple divisions and eventually contains biflagellated microgaametes which exit the microgametocyte to fertilize the macrogamete and form zygotes. Oocysts develop which are leased by rupture of the host cell and passed out with the feces to undergo sporulation, be ingested, and start its life cycle all over again.1
Generally, Eimeria sp. can be identified based on host specificity and oocyst form (see chart below), and identified postmortem using direct smears of intestinal contents or H&E stained histologic sections. Wright’s or Giemsa stain can be useful in identifying sporozoites. Simply identifying coccidian oocysts in animal feces is not enough to implicate disease since healthy animals may have large numbers. The history and clinical signs must fit with a diagnosis of coccidiosis: bloody diarrhea, weight loss, and ill thrift.1,9
Of interest, recent paleoparasitological investigation into the pre-incan to pre-hispanic contact period in northern Chile identified Eimeria macusaniensis as well as three other parasite eggs (Enterobius vermicularis, Trichostrongylus sp., and Trichuris sp.) within human coproliths (fossilized feces).3 The presence of E. macusaniensis in human fecal matter is related to the use of llamas as food, transportation, and sacrificial offerings, and underscores the importance of llamas and alpacas to societal evolution.
Table 1: Eimeria sp. by host and organs affected9
|
Animal |
Coccidia |
Organ affected/Clinical signs |
|
Birds Chickens
Turkey
Geese & ducks
Sandhill/whooping cranes Parrots |
E. acervulina E. necatrix/maxima E. brunette E. tenella E. meleagridis E. adenoeides E. meleagrimitis E. gallopavonis E. truncata E. anseris/nocens E. reichenowi E. psittaculae |
Duodenum/enteritis Jejunum/enteritis Ileum/enteritis Ceca/typhylitis Cecum Cecum, ileum Upper intestine Ileum, large intestine Kidney/anorexia, depression Intestine Disseminated Intestine |
|
Cattle
|
E. bovis/zuernii E. alabamensis |
Cecum and colon/diarrhea Small intestine |
|
Sheep |
E. ahsata/christenseni E. brakuensis E. crandallis E. ovinoidalis |
SI SI SI Cecum, colon |
|
Goats |
E. christenseni E. arloingi E. hirici E. ninakohlyak-imovea |
SI SI SI LI |
|
Equine |
E. leukarti Klossiella equi |
SI |
|
Swine |
E. debliecki |
SI (in 1-3 week old piglets) |
|
Canine |
I. canis |
Ileum, colon occasionally |
|
Feline |
I. felis |
SI, colon occasionally |
|
Mice |
Klossiella muris E. falciformis E. vermiformis E. papillata E. ferrisi |
kidney Colon Intestine Intestine Intestine |
|
Rabbit |
E. stiedae E. intestinalis E. flavescens |
Bile ducts Ileum & cecum Ileum & cecum |
|
Ferret |
E. furonis |
SI |
The sections made available to participants did not contain eosinophils in the small intestine, or bacterial colonies and ulceration within the large intestine. Attendees vigorously debated the severity of the parasitism, inflammation, and crypt hyperplasia within the small intestine and concluded that it would not be severe enough to induce ulcer formation and secondary bacterial infections further down the alimentary tract. Therefore, an additional pathogen is suspected.
Contributing Institution:
Oklahoma State University
Department of Veterinary Pathobiology
College of Veterinary Medicine
250 McElroy Hall
Stillwater, OK 74078
https://cvhs.okstate.edu/Veterinary_Pathobiology
References:
- Bowman DD. Georgis' Parasitology for Veterinarians. 10th St. Louis, MO: Elsevier; 2014: 98-103.
- Cebra CK, Valentine BA, Schlipf JW, Bildfell RJ, McKenzie E, Waitt LH, et al. Eimeria macusaniensis infection in 15 llamas and 34 alpacas. J Am Vet Med Assoc. 2007;230(1):94-100.
- De Souza MV, Da Silva LGR, Silva-Pinto V, et al. New paleoparasitological investigations form the pre-inca to hispanic contact period in northern Chile. Acta Trop. 2018;178:290-296.
- Johnson AL, Stewart JE, Perkins GA. Diagnosis and treatment of Eimeria macusaniensis in an adult alpaca with signs of colic. Vet J. 2009;179(3):465-467.
- Palacios CA, Perales RA, Chavera AE, Lopez MT, Braga WU, Moro M. Eimeria macusaniensis and Eimeria ivitaensis co-infection in fatal cases of diarrhoea in young alpacas (Lama pacos) in Peru. Vet Rec. 2006;158(10):344-345.
- Rosadio R, Londone P, Perez D, Castillo H, Veliz A, Llanco L, et al. Eimeria macusaniensis associated lesions in neonate alpacas dying from enterotoxemia. Vet Parasitol. 2010;168(1-2):116-120.
- Schrey CF, Abbott TA, Stewart VA, Marquardt WC. Coccidia of the llama, Lama glama, in Colorado and Wyoming. Vet Parasitol. 1991;40(1-2):21-28.
- Taylor MA, Coop RL, Wall RL. Veterinary Protozoology Veterinary Parasitology. 4th Hoboken, NJ: John Wiley & Sons, Inc.; 2015:129-138.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 2. 6th ed. St. Louis, MO: Elsevier; 2017:227-235.