CASE 2: 4884 A1 (4141105-00)
Signalment: Adult, neutered male, Border collie, Canis lupus familiaris
History: This case was referred to the hospital, where it presented with mild chronic hypoglycemia [blood glucose=2.8mmol/L (range: 3.6-7.0)] and high blood insulin [blood insulin= 72uIU/L (range: 5.0-40)]. Similar findings had been previously reported by the referring vet. This dog had one previous episode of seizure, which was suspected to be secondary to hypoglycemia. Ultrasonography and CT revealed a gastric fundic mass, without gastric lymphadenopathy. Cytology taken from the gastric fundus was non-conclusive. Finally, exploratory laparotomy was undertaken during which subtle thickening of the wall of the fundic stomach was noted. A transmural biopsy sample was taken during this surgery.
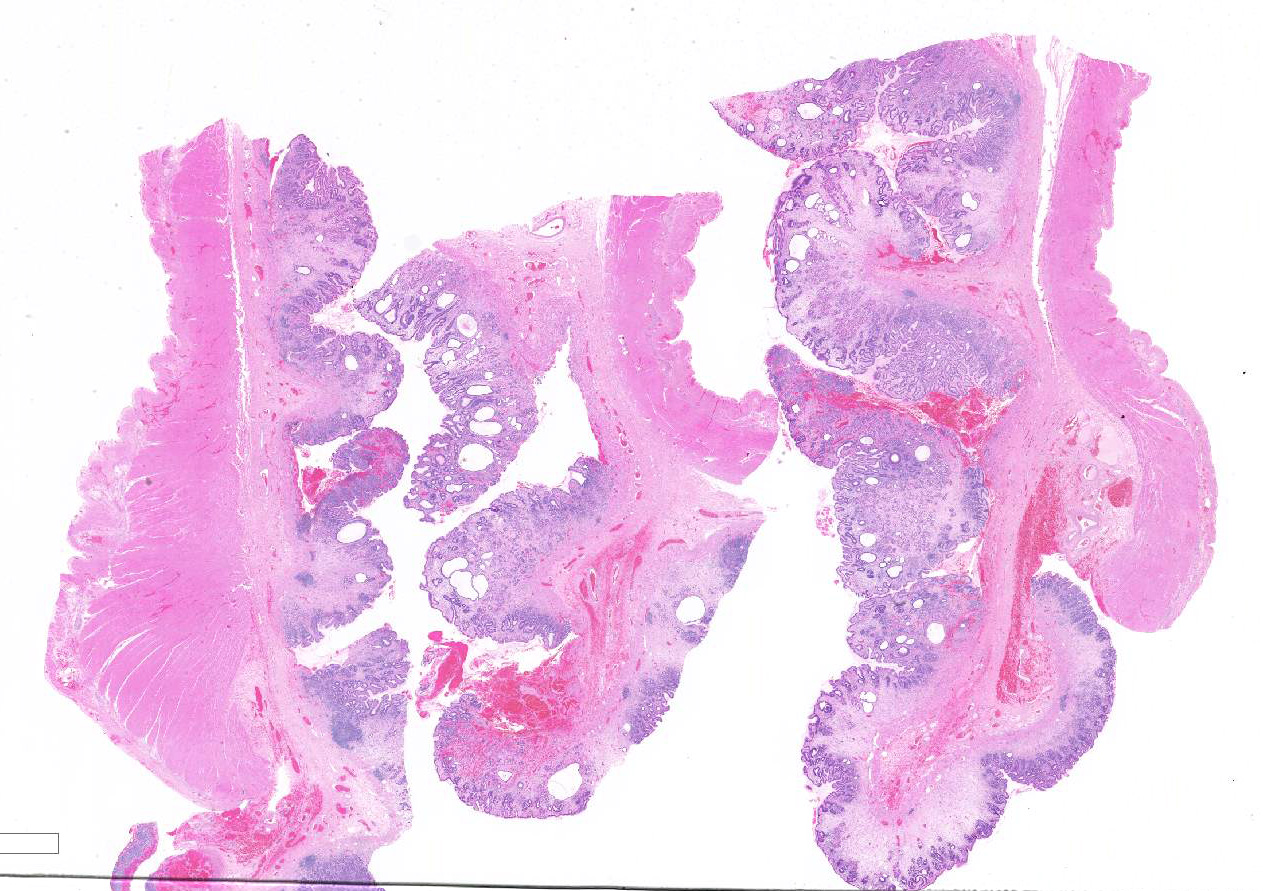
Gross Pathology: The sample submitted for histological examination was a 7cm x 7cm x 1.25cm transmural resection with diffuse, poorly defined, thickened and roughened mucosa.
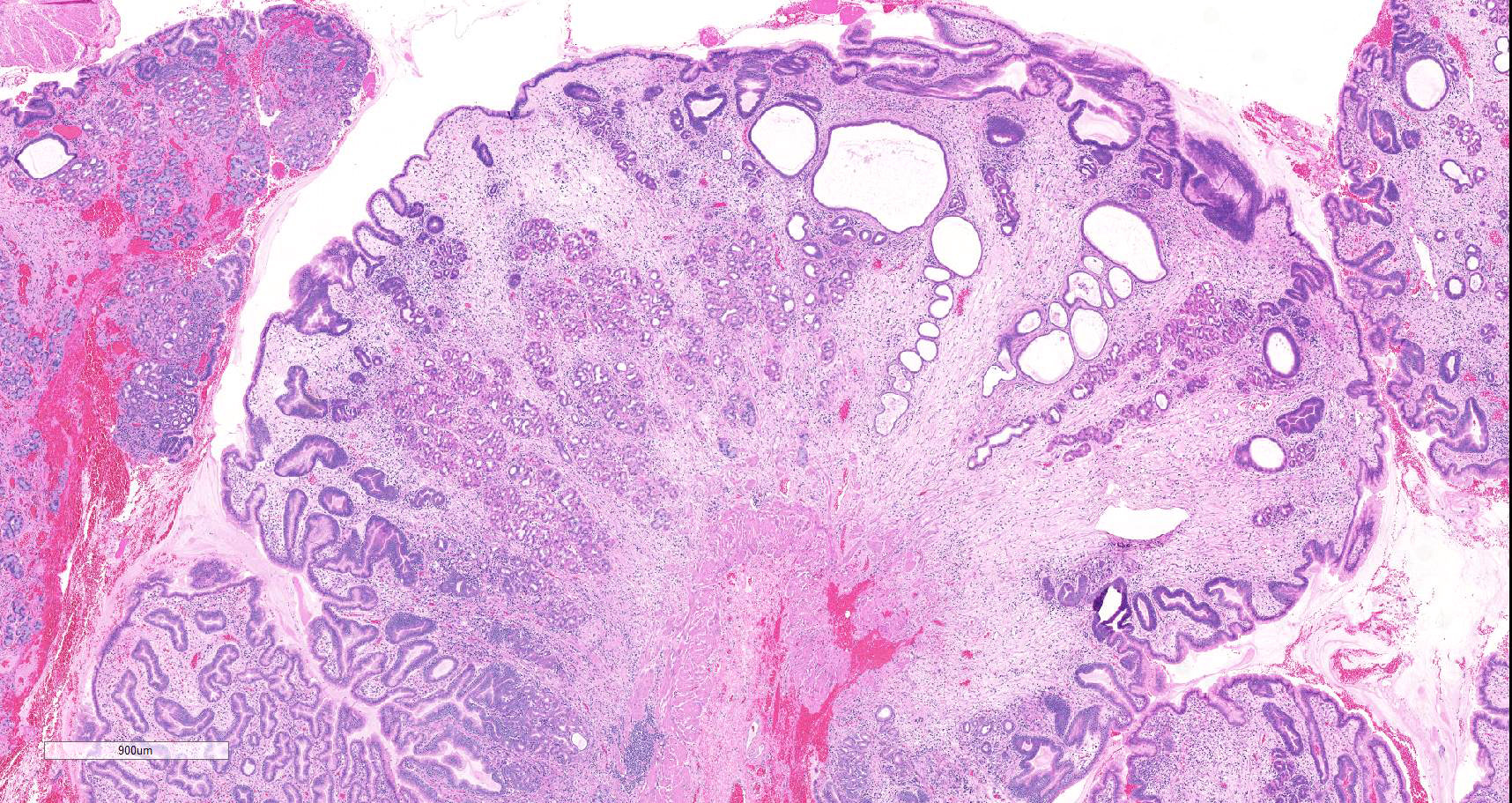
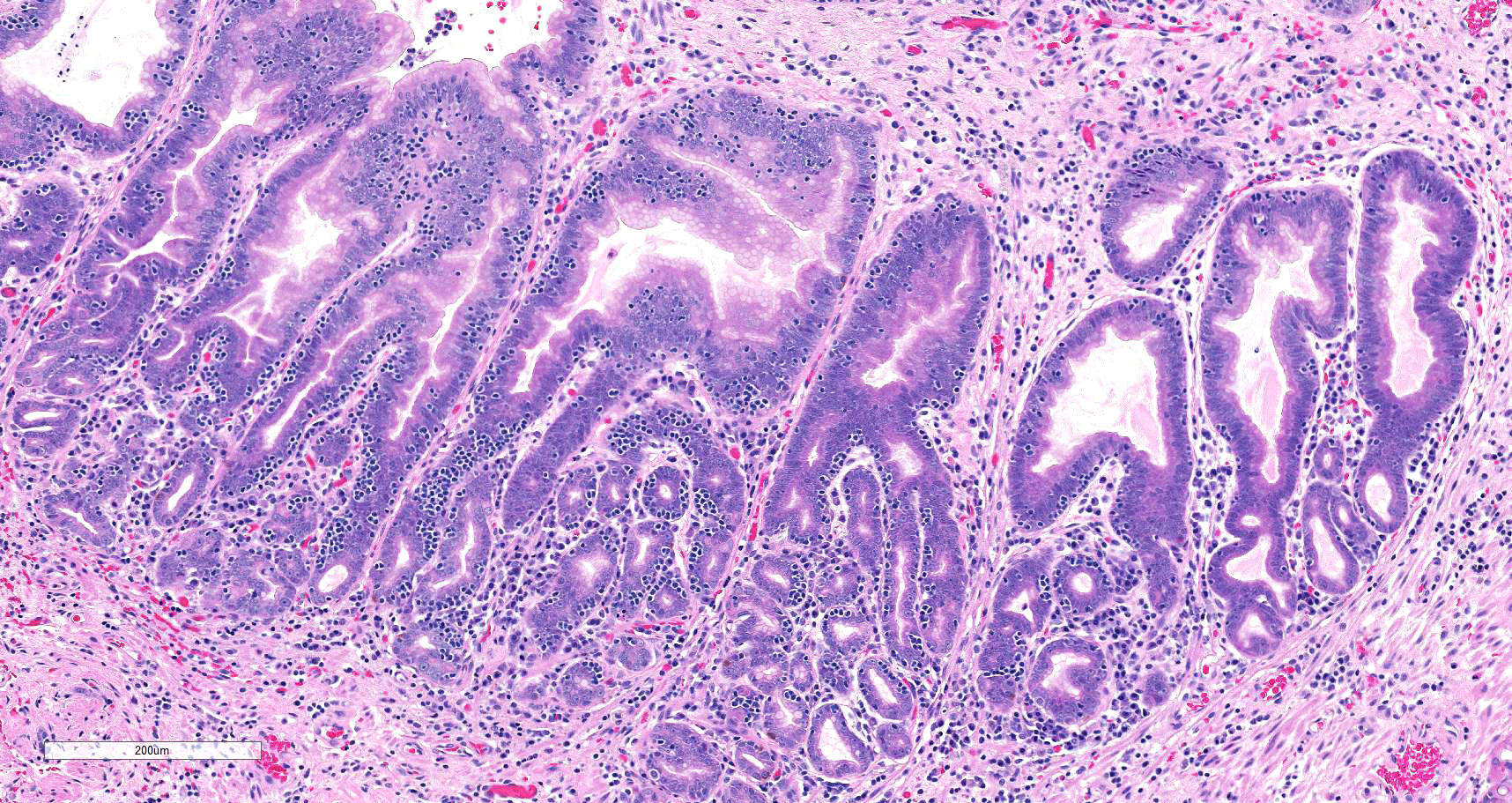
Microscopic description: There is moderate to marked, diffuse thickening of the gastric mucosa, which forms sessile and polypoid expansions into the lumen. This is due to accumulation of large numbers of glands lined mostly by a cell thick layer of columnar epithelium with basophilic to clear cytoplasm (mucus containing cells), with occasional areas where cells pile up. Moderate numbers of these glands are markedly dilated and lined by attenuated epithelium. Most of the above-mentioned glands have basal, intraepithelial infiltration by small lymphocytes. The lamina propria in these areas is infiltrated diffusely by lymphocytes and plasma cells. At the base of areas of mucosal expansion there are pockets of fundic glandular tissue (preexisting), featuring occasional intraluminal necrotic cells, and lymphoid aggregates in the lamina propria (gut associated lymphoid tissue). Additionally, there are multifocal areas of extravascular accumulation of erythrocytes in the lamina propria (hemorrhage). There is no evidence of invasion of the submucosa or the basal lamina by the hyperplastic tissue described above.
Contributor's morphologic diagnosis: Marked, diffuse, mucosal hypertrophy with chronic gastritis, mucoid metaplasia, and glandular dilation ? fundic stomach
Contributor's comment:
Gastric mucosal hypertrophy (GMH) is a canine disease that can be focal or diffuse and leads to mucosal proliferative changes. The terminology used to describe this disease can be somewhat confusing. As background, the concepts of hypertrophy and hyperplasia in both the macroscopic (gross) and microscopic settings are relevant in GMH (pathogenically and/or terminologically):6
- Hypertrophy (Greek etymology: huper ? over + trophia ? nourishment). It describes the increase in volume of a tissue or organ (gross) due to increase in parenchymal cell size (microscopic).
- Hyperplasia (Greek etymology: huper ? over + plasis ? formation). It refers to increase in volume of a tissue (gross) due to increase in the number of parenchymal cells (microscopic)
With the above in mind, it is clear that when the term hypertrophy/hypertrophic is used in the description of GMH, it refers to its gross presentation of this disease. Microscopically, GMH features both hypertrophy, and hyperplasia despite its name.
GMH can be focal or diffuse:
· Focal GMH (F-GMH) is also known as giant hypertrophic pyloric gastropathy and may feature hypertrophic antritis (i.e. hypertrophy of the pyloric antrum).4,9 It is idiopathic, relatively more prevalent than the diffuse form below, and it more frequently affects small canine breeds. In F-GMH, the proliferative lesion is located on the pyloric antrum, and consists of papillary proliferations into the lumen, which frequently lead to obstruction. Histologically, these proliferations consist of a mucosa thickened by increase in the amounts of surface epithelium foveolar tissue. Both of these are lined by epithelial cells with intracytoplasmic mucus (metaplasia). These are supported by a lamina propria that is either histologically normal, or contains plasma cells, lymphocytes, and eosinophils. It may be associated with local muscularis hypertrophy and/or mucosal erosion/ulceration.
· Diffuse GMH (D-GMH) can also be known as chronic hypertrophic gastritis or chronic giant hypertrophic gastropathy.4,9 It is also idiopathic although an immune mediated pathogenesis is suspected. DS-GMH is relatively rarer than F-GMH and can affect a range of dog breeds. Breeds overrepresented include Basenji, beagle, boxer and bull terriers. It is associated with vomiting, diarrhea, weight loss, protein-losing gastropathy, and hypoproteinemia. Its presentation is similar to that described for human diseases with Menetrier disease (more on this below). The gastric mucosa is diffusely thickened to form ?cerebriform? folds that do not resolve upon stretching of the gastric wall. These may involve an area of 4?10cm only (which contrasts with the use of the term diffuse in the terminology above). Microscopically, there is hypertrophy and hyperplasia of the mucosa to form folds that may or may not contain submucosa and muscularis layers. The hyperplastic mucosa is composed of foveolar and glandular tissue, and there is progressive loss of parietal cells, that are replaced by variably differentiated mucus containing cells. A distinctive feature is the presence of inflammatory infiltration -and occasionally edema- of the lamina propria, which is mononuclear (i.e. lymphocytes and plasma cells with rarer macrophages). Partial gastrectomy can be curative.10
Overall, the histological presentation of the sample of stomach in this case is consistent with D-GMH, as this diagnosis is supported by the location (i.e. fundic stomach), gross presentation, and histological features. A striking feature of this presentation are the intraepithelial lymphocytes. More investigation would be required to ascertain their role (e.g. immunohistochemical characterization), but these are suspected to be secondary to inflammation. One puzzling aspect of this presentation is the hypoglycemia, which is not a feature of the presentation of any of the diseases above described. It is therefore possible that this feature was not associated with the gastric lesion.
Parallelism between GMH (a.k.a. chronic hypertrophic gastritis or chronic giant hypertrophic gastropathy) and human Menetrier disease is often drawn. Menetrier disease is a hypertrophic gastropathy also characterized by ?cerebriform? thickening of the gastric fundus/body mucosa secondary to excessive production of transforming growth factor alpha (TGF-alpha).8 It affects adults (30-60y) most frequently, but a self-limiting pediatric form is reported (usually following respiratory infection). It is characterized by foveolar epithelial hyperplasia (with mucus containing cells), parietal and chief cell atrophy, and mild to moderate inflammation. Interestingly, striking intraepithelial lymphocytosis is present in some cases, in consistency with the canine D-GMH case presented here. Menetrier disease in adult humans is associated with increased risk of gastric adenocarcinoma.
Canine gastric adenocarcinoma (GA) is a differential diagnosis to consider for this presentation.9 There are several features of GA that contrast with the presentation
noted in this case, however. Grossly, GAs cause loss of the rugal aspect of the mucosal surface, may be ulcerated (>50% of cases), and are frequently located in the pylorus/antrum. Histologically, they frequently have severe desmoplasia, and are heavily infiltrative, with frequent invasion of the lamina propria progressing into the muscularis and serosa to form a transmural lesion. Also, lymphatic invasion and metastasis to local lymph nodes and other distant organs (especially lung, liver and adrenal glands) are common occurrences in GA. None of these were noted in this case.
Contributing Institution:
Easter Bush Pathology
Easter Bush Veterinary Centre
Roslin, Midlothian, EH25 9RG, UK https://www.ed.ac.uk/vet/services/easter-bush-pathology
JPC diagnosis:
Stomach: Gastric hypertrophy, chronic, diffuse, severe, with mucoid metaplasia, marked glandular atrophy and loss, and moderate lymphocytic inflammation.
JPC comment:
First described in 1888 by French pathologist Pierre Menetrier, this disease was first characterized by ?sheet-like polyadenomas? of the gastric mucosa affecting the proximal portion of the stomach (body and fundus) but sparing the distal stomach (antrum). Many of his findings remain characteristic for the eponymous Menetrier's disease.3
The contributor provided a good review of this entity. In humans, Menetrier's disease is also known as hyperplastic gastropathy, or giant rugal hypertrophy. A familial link has been rarely documented but shows no regular association with other diseases. Interestingly, cytomegalovirus is present in approximately 70% of cases in affected children, and Helicobacter pylori is detected in almost 90% of adults affected.2
Giant hypertrophic gastritis, or Menetrier-like disease, has been reported in an Old English sheepdog, a Jack Russell terrier, and a Boxer. A Menetrier-like disease has been reported in association with gastric carcinoma in a West Highland white terrier, and gastric adenocarcinoma in Cairn terrier littermates. The contributor previously mentioned the breed specific form of gastric hypertrophy in Baseneji, though that histologic appearance differs from Menetrier-like disease exhibiting only infrequent glandular cysts and a concurrent lymphoplasmacytic gastroenteritis. A recent report of Menetrier-like disease in a domestic shorthair cat found a predominant mucosal hyperplasia, dilated gastric glands, a mild interstitial fibrosis and inflammatory infiltrates.1
A hypertrophic gastropathy similar to Menetrier's disease has been reported in transgenic mice. A line of mice was created that overexpressed transforming growth factor a (TGF-a) in the stomach. The mice exhibited dramatic structural and functional lesions of the glandular stomach, including severe adenomatous hyperplasia and a lack of detectable gastric acid. It remains possible that TGF-a plays an important role in the development of pathology similar to Menetrier's disease.7
Important differentials to consider for diffuse hypertrophic gastropathy include Menetrier's disease, hypertrophic lymphocytic gastritis, hypertrophic hypersecretory gastropathy, and Zollinger-Ellison syndrome. Hypertrophic lymphocytic gastritis can be differentiated by diffuse and severe inflammation with numerous intraepithelial lymphocytes. Hypertrophic hypersecretory gastropathy differs from Menetrier's disease by exhibiting hyperplasia of both the foveolar epithelium and oxyntic glands, with cystic dilatation of the gastric glands possible. Zollinger-Ellison syndrome is characterized by a lack of foveolar hyperplasia, but with ectopic gastrin secretion (gastrinoma) with increased gastric acid secretion, intractable peptic ulcers, diffusely thickened gastric folds, and diffuse parietal cell hyperplasia and hypertrophy.5
References:
1. Barker EN, Holdsworth AS, Hibbert A, Brown PJ, Hayward NJ. Hyperplastic and fibrosing gastropathy resembling Menetrier disease in a cat. Journal of Feline Medicine and Surgery. 2019;5(2):1-7.
2. Campbell F, Lauwers GY. Tumors of the Esophagus and Stomach. In: Fletcher CDM ed. Diagnostic Histopathology of Tumors, 4th Ed. Vol 1, Part 2. Philadelphia, PA: Elsevier. 2013:421-422.
3. Coffey RJ, Tanksley J. Pierre Menetrier and his disease. Transactions of the American Clinical and Climatological Association. 2012; 123:126-133.
4. Gelberg H: Alimentary System and the Peritoneum, Omentum, Mesentery and Peritoneal cavity. In: Pathologic basis of veterinary disease, ed. Zachary JF, 6th ed., pp. 324-411. Elsevier, St Louis, Missouri, USA, 2017.
5. Huh WJ, Coffey RJ, Washington MK. Menetrier's Disease: Its mimickers and pathogenesis. Journal of Pathology and Translational Medicine. 2016; 50:10-16.
6. Margaret A M, Zachary JF: Mechanisms and morphology of cellular injury, adaptation, and death. In: Pathologic basis of veterinary disease, ed. Zachary JF, 6th ed., pp. 23-25. Elsevier, St Louis, Missouri, USA, 2017.
7. Takagi H, Jhappan C, Sharp R, Merlino G. Hypertrophic gastropathy resembling Menetrier's disease in transgenic mice overexpressing transforming growth factor a in the stomach. Journal of Clinical Investigation. 1992; 90:1161-1167.
8. Turner JR: The gastrointestinal tract. In: Pathologic basis of disease, eds. Kumar V, Abbas AK, Aster JC, pp. 749-819. Elsevier Saunders, Philadelphia, USA, 2015.
9. Uzal F, Plattner BL, Hostetter JM: Alimentary system. In: Pathology of domestic animals, ed. M GM, 6th ed., pp. 1-257. Elsevier, St Louis, Missouri, USA, 2016.
10. Vaughn DP, Syrcle J, Cooley J: Canine giant hypertrophic gastritis treated successfully with partial gastrectomy. J Am Anim Hosp Assoc 50: 62-66, 2014.