WSC 22-23
Conference 11
Case II:
Signalment:
An adult, captive, female giant spider crab (Macrocheira kaempferi; Temminck, 1836).
History:
This giant spider crab (Macrocheira kaempferi) was found deceased after a short period of anorexia. Other decapods in the tank had shown signs of “shell disease syndrome”.
Gross Pathology:
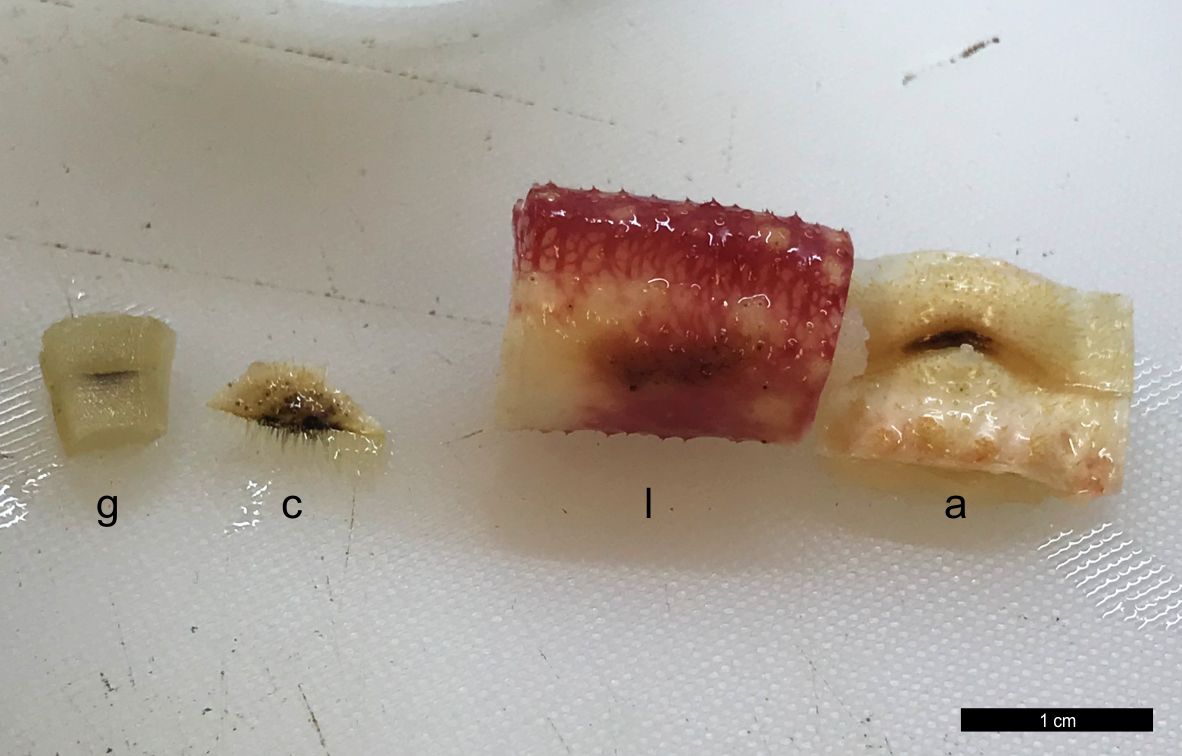
At necropsy, the main gross pathologic findings were multifocal, variably sized, dark brown to black, depressed skin foci in the apron, limb, and carapace.
Laboratory Results:
Multiple bacterial cultures of skin and internal organs from other similarly affected and deceased crabs yielded Vibrio sp. (1+), Shewanella putrefaciens (1+), and Corynebacterium sp. (1+).
Microscopic Description:
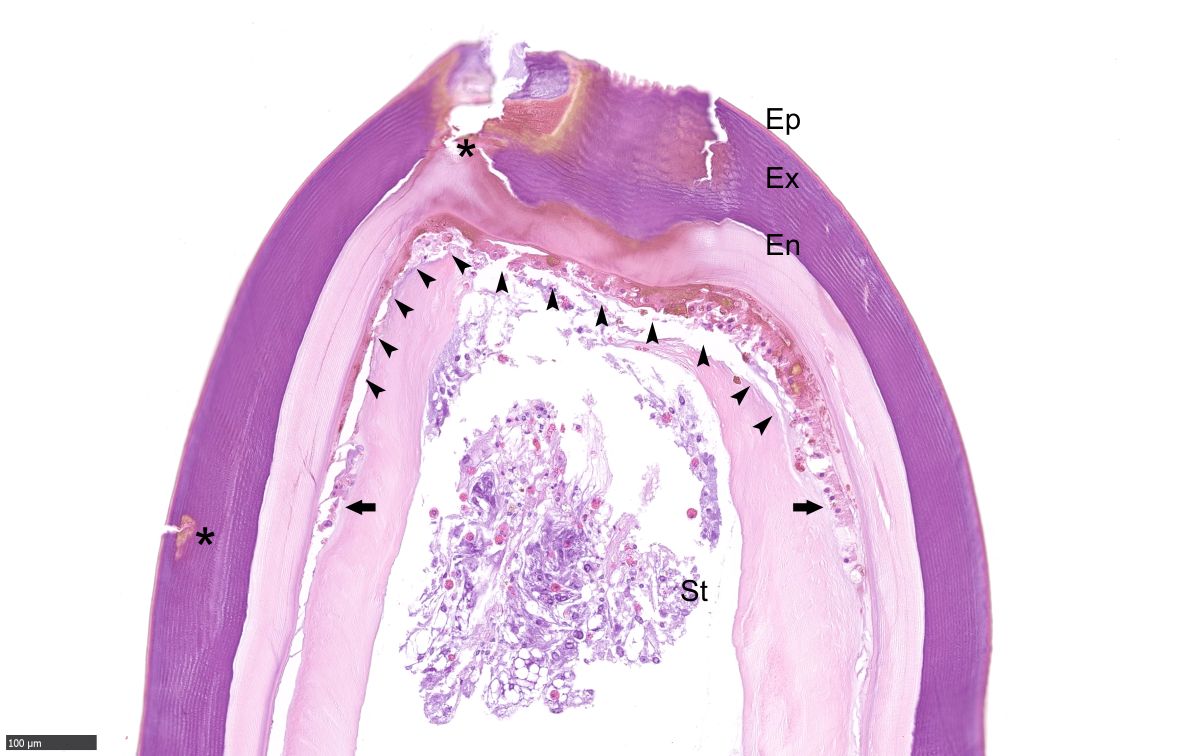
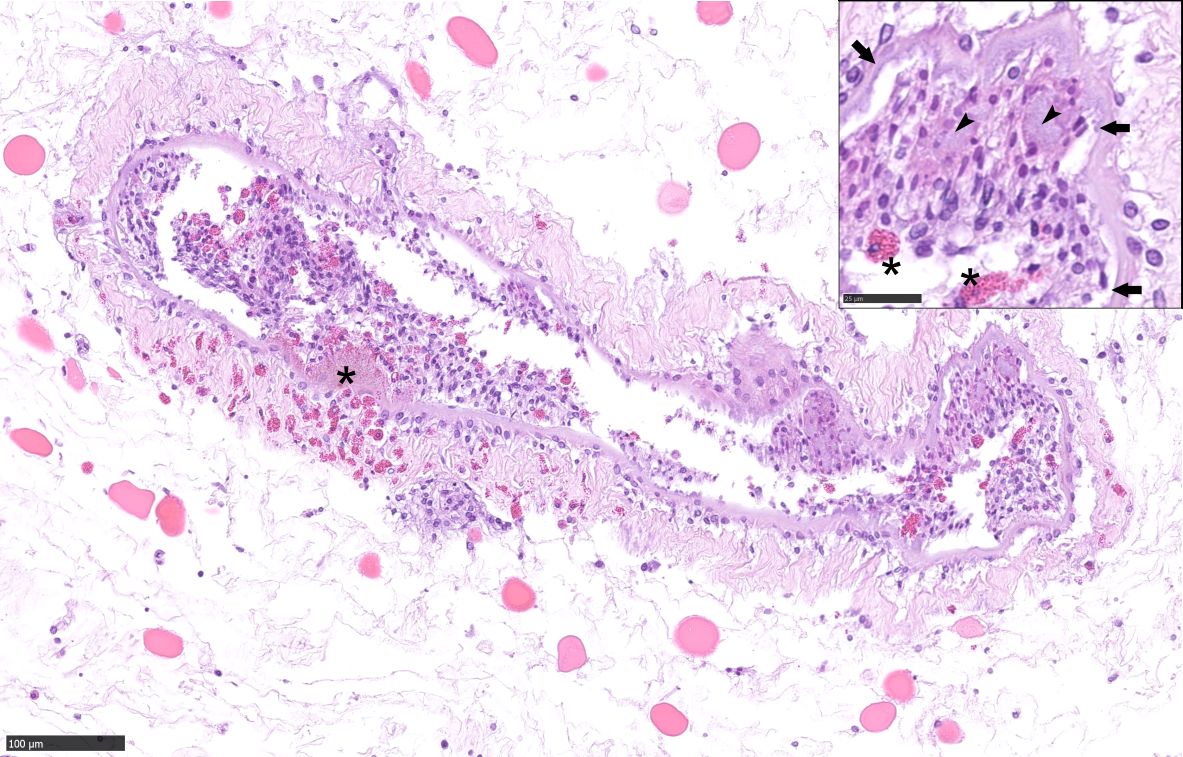
Apron, Carapace, Limb: Multifocally, the epi-, exo- and endo-cuticle as well as the membranous layer are eroded and ulcerated. Variable amounts of proteinaceous material and hemocyte debris expand and disrupt the cuticle, membranous layer, and epidermis. Multifocally, the epidermis and dermis are infiltrated by granular and reactive agranular hemocytes, occasional melanized cells, karyorrhectic cellular debris and extracellular and intraphagocytic bacteria. Inflammatory infiltrates are often more prominent at the setae. Some of these hemocytes infiltrate the connective tissue amid myocytes and variably infiltrate and disrupt vascular structures, more prominently within the deep soft tissue in the apron. Rare hemocyte nodule formation is noted. Hemolymph sinuses exhibit occasional eosinophilic globules.
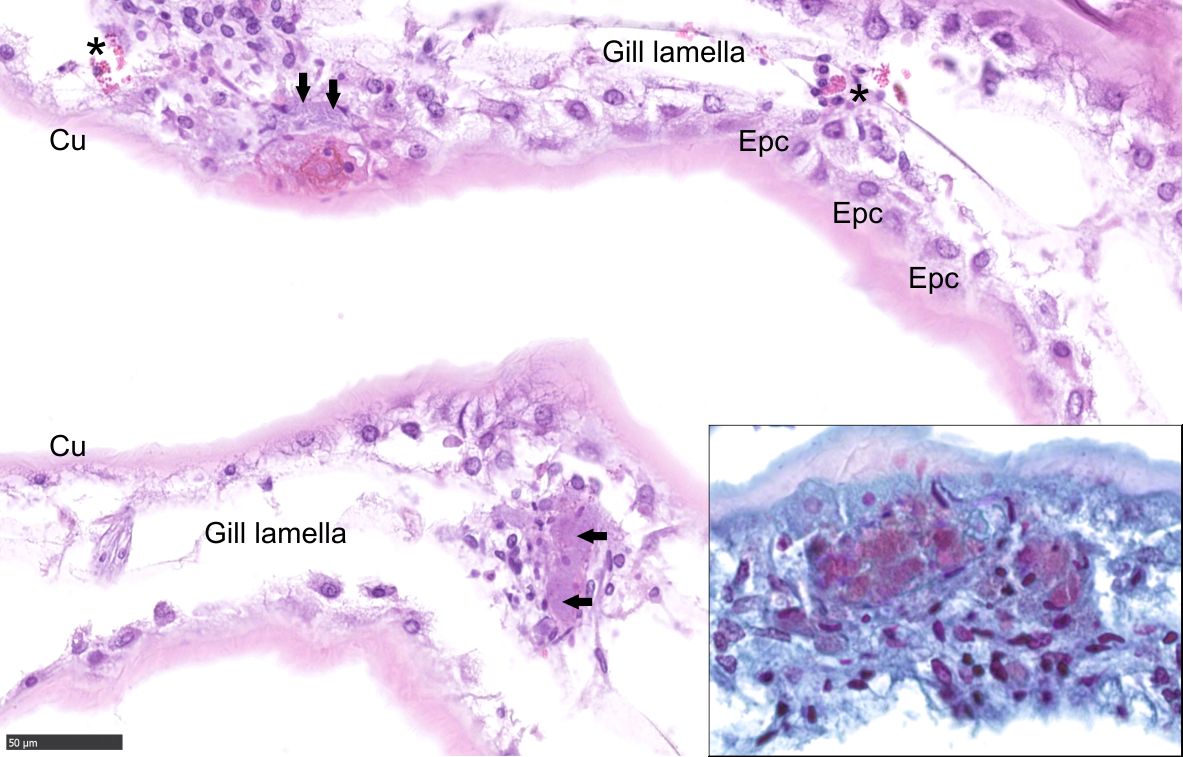
Gill: Multifocally, the gills are infiltrated and disrupted by granular and agranular hemocytes, cellular debris, proteinaceous fluid, and intraphagocytic and intravascular bacteria.
Contributor’s Morphologic Diagnoses:
Apron, Carapace, Limb: Mild to marked, multifocal, subacute erosivo-ulcerative hemocyte epidermitis and dermatitis/hypodermitis with intralesional and intravascular gram-negative bacteria, melanization, and vasculitis.
Gill: Moderate, multifocal, subacute hemocyte branchitis with intralesional and intravascular gram-negative bacteria.
Contributor’s Comment:
Based on the clinical history and bacterial culture results from similarly affected and deceased conspecifics, the gross and histopathologic findings in this crab are strongly suggestive of black spot shell disease syndrome (SDS) and hemocoelic bacterial invasion (septicemia).3,12,15
Macrocheira kaempferi (infraorder Brachyura, order Decapoda) is the largest of extant Arthropoda. This species occurs along the Pacific coast of Japan, at depths ranging from 50 to 400 m.5 Although M. kaempferi has been introduced into aquaria worldwide, knowledge on health and disease aspects in the species is very limited.1,8
SDS is regarded as a multifactorial syndrome and is one of the main concerns on crabs newly introduced into aquaria.1,9 Two potential forms of SDS have received attention. These include a) the “classic” form, which is centered around chitin degradation and has been reported in many crustaceans, including lobsters, crabs and shrimp,7,13 and b) an “epizootic” form, in which chitin degradation is of less significance and primarily affects lobsters.2 Various environmental stressors14 and opportunistic chitinolytic bacteria (particularly in the classic form), such as Vibrio sp, Photobacterium sp, Aeromonas sp, Alteromonas sp, Pseudoalteromonas sp, Clostridium sp, Cytophaga sp, Chromobacteria sp., protozoans and/or fungi play central roles.4,14,10 High occurrence is linked to polluted environments.14
Grossly, SDS is characterized by exoskeletal erosions, pitting, and discoloration, including typical black spots resulting from melanization.4 Lesions vary from mild (restricted to the exoskeleton), to severe, where injury may extend through the entire shell and into the soft tissues, often resulting in extensive ulcers, fractures, and autotomy.6 Lesions may initiate via destruction of the epicuticular layer, by proteolytic and lipolytic microbial activities, predatory or cannibalistic attacks, chemical injury or the abrasive action of sediment and/or articulated body parts; the setal pores are often affected first and may favor internal invasion.7 Microscopically, there is progressive erosion, ulceration and disruption of the exoskeleton and epidermis. The inflammatory response may include exudation of proteinaceous material, hemocyte debris, infiltration of granular and reactive agranular hemocytes, melanized cells, and extracellular and intraphagocytic bacteria; these changes were observed in the present case. In severe cases, bacteria may extend into the internal viscera and mortality can ensue as result of primary or secondary infection.12 In the present case, traumatic skin injury by conspecific males likely predisposed to opportunistic bacterial colonization, resulting in eventual hemocoelic bacterial invasion, septicemia, and death.11,12
SDS is difficult to control and eradicate, despite occasional remission after molting.3 Although fallible, treatment methods have been proposed to contain disease progression.1 In conclusion, the diagnosis of SDS relies upon visualization of typical gross findings combined with histopathology and microbiologic analysis.
Contributing Institution:
Texas A&M Veterinary Medical Diagnostic Laboratory (TVMDL), 483 Agronomy Rd, College Station, TX 77843, USA. https://tvmdl.tamu.edu/
JPC Diagnosis:
Exoskeleton and gills: Dermatitis and branchitis, ulcerative, hemocytic, chronic, multifocal, moderate, with melanization and cuticular epithelial hyperplasia, vasculitis, and intraphagocytic and extracellular bacteria.
JPC Comment:
This week’s moderator, Dr. Elise LaDouceur, editor of the recently published textbook Invertebrate Histology, described the invertebrate immune system, which lacks an adaptive arm and features hemocytes as the effector cell in most species. In crustaceans and other arthropods, hemocytic recognition of pathogen- or damage-associated molecular patterns leads to adhesion, degranulation, and phagocytosis, or for larger pathogens, encapsulation or nodulation with subsequent melanization. The last step leads to formation of reactive oxygen and nitrogen species and is the underlying cause of the eponymous “black spots” in this syndrome.
Chitin is a biopolymer which makes up 70% of the organic fraction of crustacean exoskeletons and is rapidly degraded by microbes after crustacean molting or death.14 Chitinolytic bacteria travel along a chitin oligosaccharide gradient, adhere to and form biofilms on chitinous surfaces, express chitin catabolic cascade genes, and produce chitinase and beta glucosaminidases responsible for chitin breakdown.14 These mechanisms allow the bacteria to use chitin as a source of nitrogen and carbon, which are absorbed through chitoporins.14
The healthy cuticle of crustaceans is resistant to such chitinolytic activity because the most superficial layer - the epicuticle - is nonchitinous and composed primarily of proteolipid material.12 Disruption of the epicuticle may be mediated by trauma or abrasion, as mentioned by the contributor, or by lipolytic actions of other microbes.12 Older crustaceans are more susceptible to degradation as they molt and replace their exoskeleton less frequently.14 Once the epicuticle is disrupted, the chitinous pro-cuticle is exposed and can be colonized by chitinolytic bacteria, which are usually part of a mixed population of microbes which may produce other enzymes, scavenge liberated nutrients, or prey on other microbes.14
As the contributor describes, severe lesions can lead to secondary hemocoelic invasion and septicemia. Internal lesions associated with SDS were previously described in a study of shell-disease affected edible crabs, Cancer pagarus.12 In the gills, there were hemocytic infiltrates and nodules which frequently occluded the hemal sinuses, the thin epithelium was occasionally breached and sealed by melanized hemocytic plugs, and nephrocytes at lamellar tips were swollen with abundant dark brown material within a central cytoplasmic vacuole.12 Hepatopancreatic tubules exhibited variable necrosis and hemocytic nodules in hemal spaces.12 Hemocytic nodules were also observed in the heart.12 In general, the severity of the internal lesions mirrored the extent of the shell lesions.12
References:
- AZA Aquatic Invertebrate Taxon Advisory Group. Japanese spider crab care manual. Silver Spring, MD. 2014; Association of Zoos and Aquariums.
- Chistoserdov AY, Smolowitz R, Mirasol F, Hsu A. Culture-dependent characterization of the microbial community associated with epizootic shell disease lesions in American lobster, Homarus americanus. J Shellfish Res. 2005;24: 741-747.
- Noga EJ, Hancock AL. Crustaceans. In: Lewbart GA, ed. Invertebrate medicine. John Wiley & Sons; 2011.
- Noga EJ, Smolowitz R, Khoo LH. Pathology of shell disease in the blue crab, Callinectes sapidus Rathbun (Decapoda: Portunidae). J Fish Dis. 2000;23:389-399.
- Nonaka M, Iwahashi Y. Some aspects and problems on fisheries of giant spider crab Macrocheira kaempferi (Temmick). Aquac Sci. 1987;35:21-26.
- Smolowitz R, Chistoserdov AY, Hsu A. A description of the pathology of epizootic shell disease in the American lobster, Homarus americanus, H. Milne Edwards 1837. J Shellfish Res. 2005;24:749-756.
- Smolowitz RM, Bullis RA, Abt DA. Pathological cuticular changes of winter impoundment shell disease preceding and during intermolt in the American lobster, Homarus americanus. Biol Bull Woods Hole. 1992;183:99-112.
- Stegeman N, Allender M, Arnold J, Bonar CJ. Aquatic Invertebrates. Exot Anim Lab Diagn. 2020:383-408.
- Stewart JE. Infectious diseases of marine crustaceans. In: Couch JA, Fournie JW, eds. Pathobiology of marine and estuarine organisms. Boca Raton, FL, USA: CRC Press; 1993.
- Tlusty MF, Smolowitz RM, Halvorson HO, DeVito SE. Host susceptibility hypothesis for shell disease in American lobsters. J Aquat Anim Health. 2007;19: 215-225.
- Ueda R, Yasuhara T, Sugita H, Deguchi Y. Gut microflora of the Japanesese giant crab Macrocheira kaempferi. Nippon Suisan Gakki. 1989;55:181.
- Vogan CL, Costa-Ramos C, Rowley AF. A histological study of shell disease syndrome in the edible crab Cancer pagurus. Dis Aquat Organ. 2001;47:209-217.
- Vogan CL, Llewellyn PJ, Rowley AF. Epidemiology and dynamics of shell disease in the edible crab Cancer pagurus: a preliminary study of Langland Bay, Swansea, UK. Dis Aquat Organ. 1999;35:81-87.
- Vogan CL, Powell A, Rowley AF. Shell disease in crustaceans - just chitin recycling gone wrong? Environ Microbiol. 2008;10:826-835.
- Wang W. Bacterial diseases of crabs: a review. J Inverteb Pathol. 2011;106:18-26.