Signalment:
Gross Description:
Histopathologic Description:
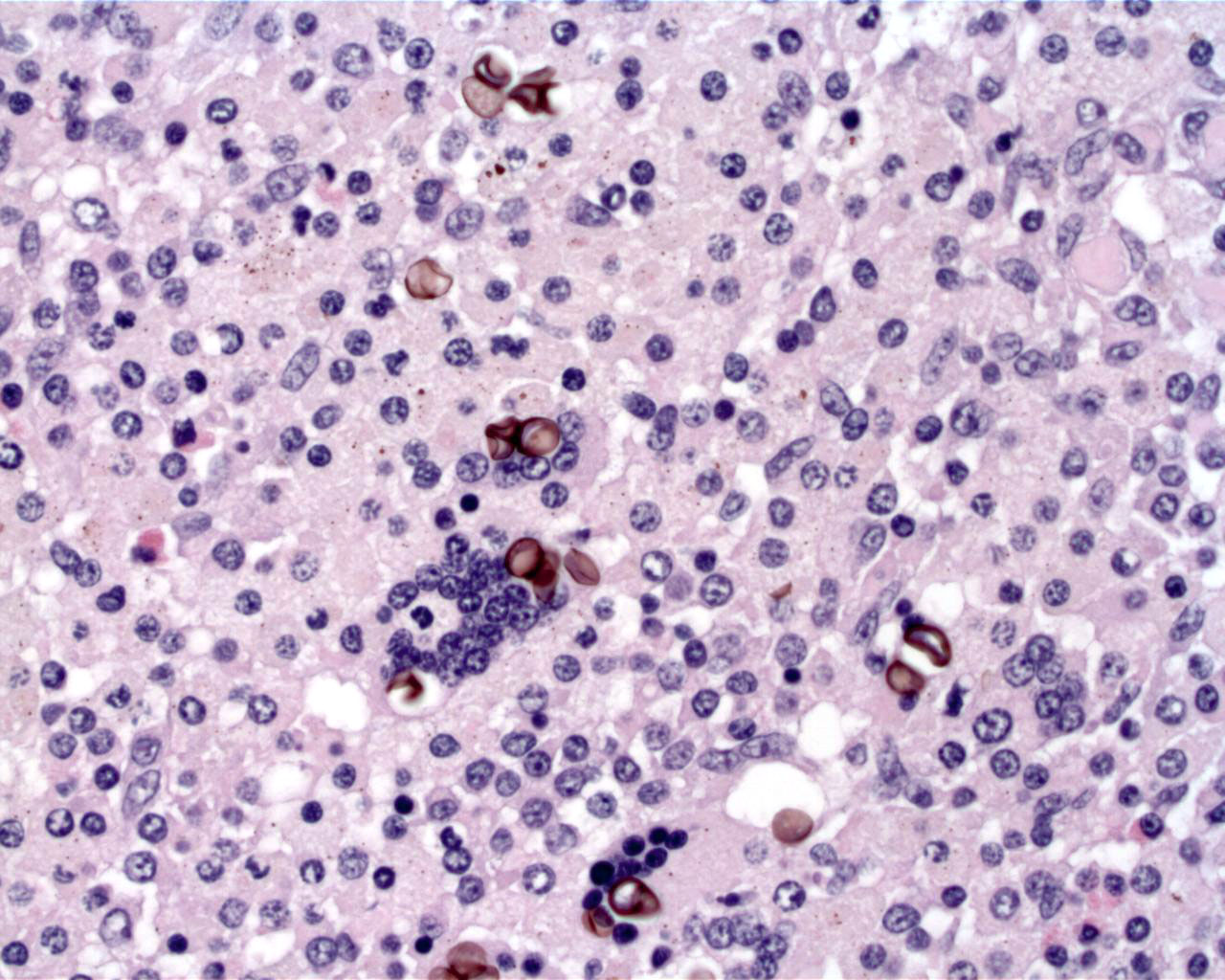
Acid fast: There are scattered, positive bacilli throughout granulomas.
Morphologic Diagnosis:
Lab Results:
Culture of the lung grew Mycobacterium gordonae
Condition:
Contributor Comment:
In amphibians, Mycobacterium induces granulomas similar to those precipitated by M. tuberculosis in humans. Key differences for amphibians include few multinucleated giant cells, a lack of mineralization, and a propensity for development in the skin4. Additionally, cytology and histology of amphibian lesions reveal numerous acid fast organisms, in contrast to the paucibacillary lesions of human TB. Cutaneous mycobacteriosis in the amphibian is most often associated with M. marinum, another water-borne mycobacterial species. The initial inflammatory response is disorganized and histiocytic and can be seen histologically by 2 weeks post-infection1. Mature granulomas composed of sheets of interdigitating epithelioid macrophages surrounded by a fine, fibrous capsule are seen by 8 weeks post-infection. Caseation of the core may occur in some granulomas3 effectively walling off the bacteria from the host immune system. In this way, latent infection may be maintained.
JPC Diagnosis:
Conference Comment:
The initiation of the cell-mediated immune response consists of activated macrophages secreting interleukin-12, which in turn skews the immune system to secrete interferon-γ and interleukin-2 by CD4+ T-helper-1 lymphocytes.3 Tumor necrosis factor-_ and interferon-γ act together to promote the formation of the tuberculoid granuloma.3
Granulomas in the amphibians induced by Mycobacterium marinum share many features with human tuburculosis granulomas.1 They contain mature macrophages, epithelioid cells, and extracellular matrix components. Yet caseation does not appear to be a feature in frog granulomas even though it is seen in M. marinum infections of humans, goldfish and toads.1
Hyphae due to the systemic chromoblastomycosis were not present in all lung sections, so the entity was not discussed by the contributor. Chromoblastomycosis can be caused by a wide variety of brown pigmented, dematiaceous fungi, and infections have been seen in mammals and amphibians.2 In amphibians the infection is often systemic with lesions in the skin, liver, lungs, and kidneys of stressed animals.2
References:
2. Bube A, Burkhardt E, Weiss R: Spontaneous chromomycosis in the marine toad (Bufo marinus). J Comp Path 106:73-77, 1992
3. Caswell JL, Williams KJ: Respiratory system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 2, pp. 606-610. Elsevier Limited, St. Louis, MO, 2007
4. Green, DE: Infectious etiologies Mycobacteria Mycobacteriosis. In: Amphibian Medicine and Captive Husbandry, eds. Wright KM, Whitaker BR, pp. 427-429. Kreiger Publishing Co., Malabar, FL, 2001
5. Rastogi N, Legrand E, Sola C: The mycobacteria: an introduction to nomenclature and pathogenesis. Rev Sci Tech 20:21-54, 2001
6. Shively JN, Songer JG, Prchal S, Keasey MS 3rd, Thoen CO: Mycobacterium marinum infection in Bufonidae. J Wildl Dis 17:3-8, 1981
7. Taylor SK, Williams ES, Thorne ET, Mills KW, Withers DI, Pier AC: Causes of mortality of the Wyoming toad. J Wildl Dis 35:49-57, 1999