CASE I: 576/20 (JPC 4161128)
Signalment:
3 year old, female, dog, German shepherd
History:
The animal began to move with difficulty two months before death. Neurological and CT examination raised the suspicion of the cauda equine syndrome. The animal underwent surgery that, like postoperative recovery, went smoothly. Two weeks after the surgery, the animal again began to move with difficulties and uncoordinated. Clinical examination revealed pharyngitis, rhinitis, chorioretinitis, and partial retinal ablation. Several differential diagnoses have been proposed that included neoplasia, bacteremia, fungal infection, autoimmune diseases and vascular anomalies (hypertension, vasculitis). Steroid therapy has been started but the animal developed convulsive seizures after two days and due to the deterioration of the condition, the animal was euthanized at the owner request.
Gross Pathology:
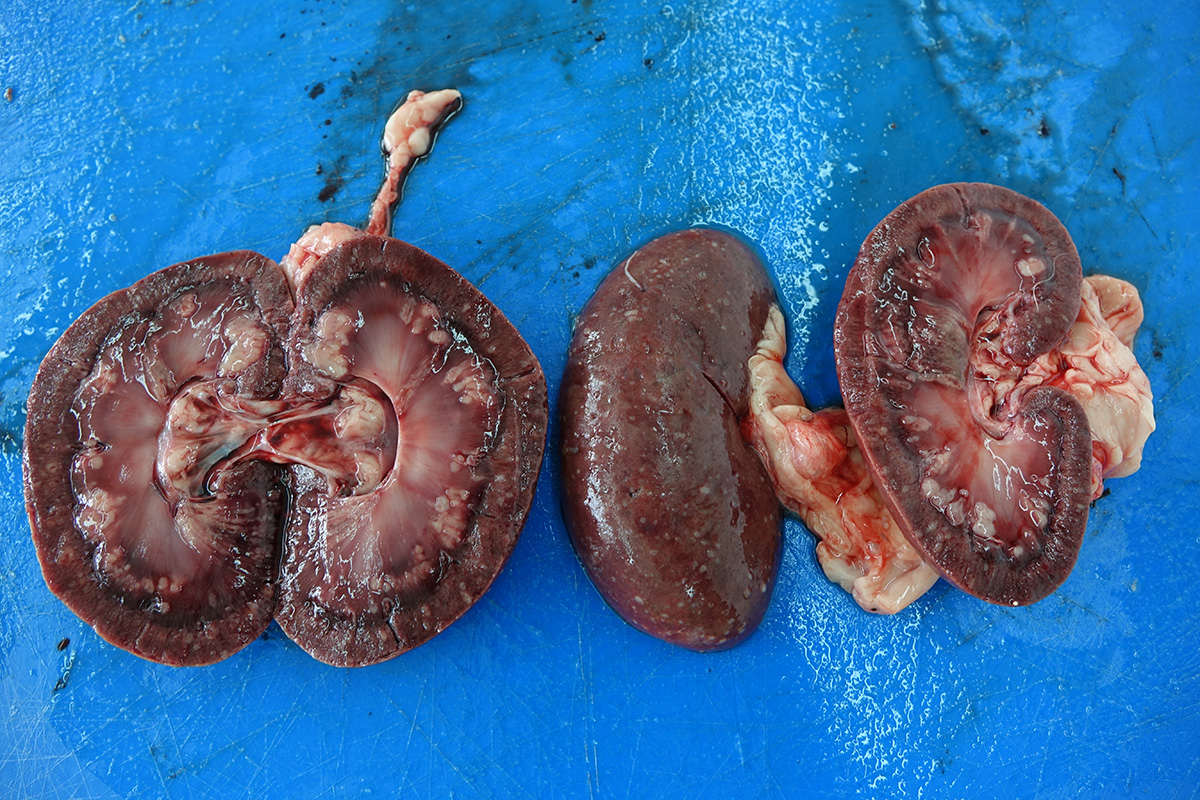
The necropsy revealed severe bilateral granulomatous nephritis. Multiple splenic nodules interpreted as granulomas or abscesses were found. In lungs, diffuse congestion, edema, marginal emphysema and multifocal to coalescing hemorrhages. In heart, eccentric hypertrophy of the left ventricle and multifocal epicardial hemorrhages were noted. In liver and brain severe congestion were present.
Laboratory results:
E. coli and Cryptococcus sp. were isolated by postmortem microbiological examination of kidney samples. Mucicarmine special staining reveal positive staining of yeasts with narrow based budding.
Microscopic Description:
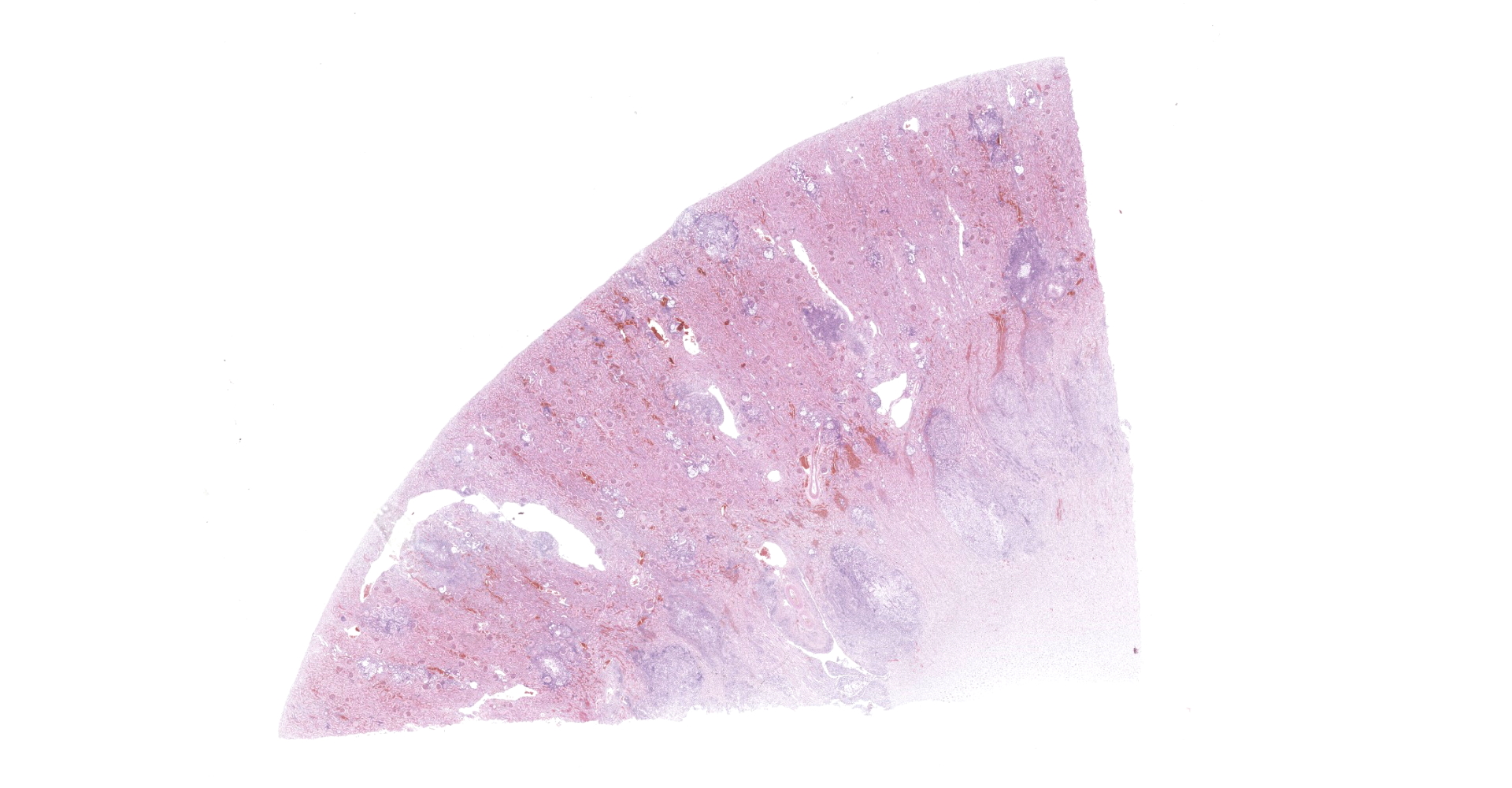
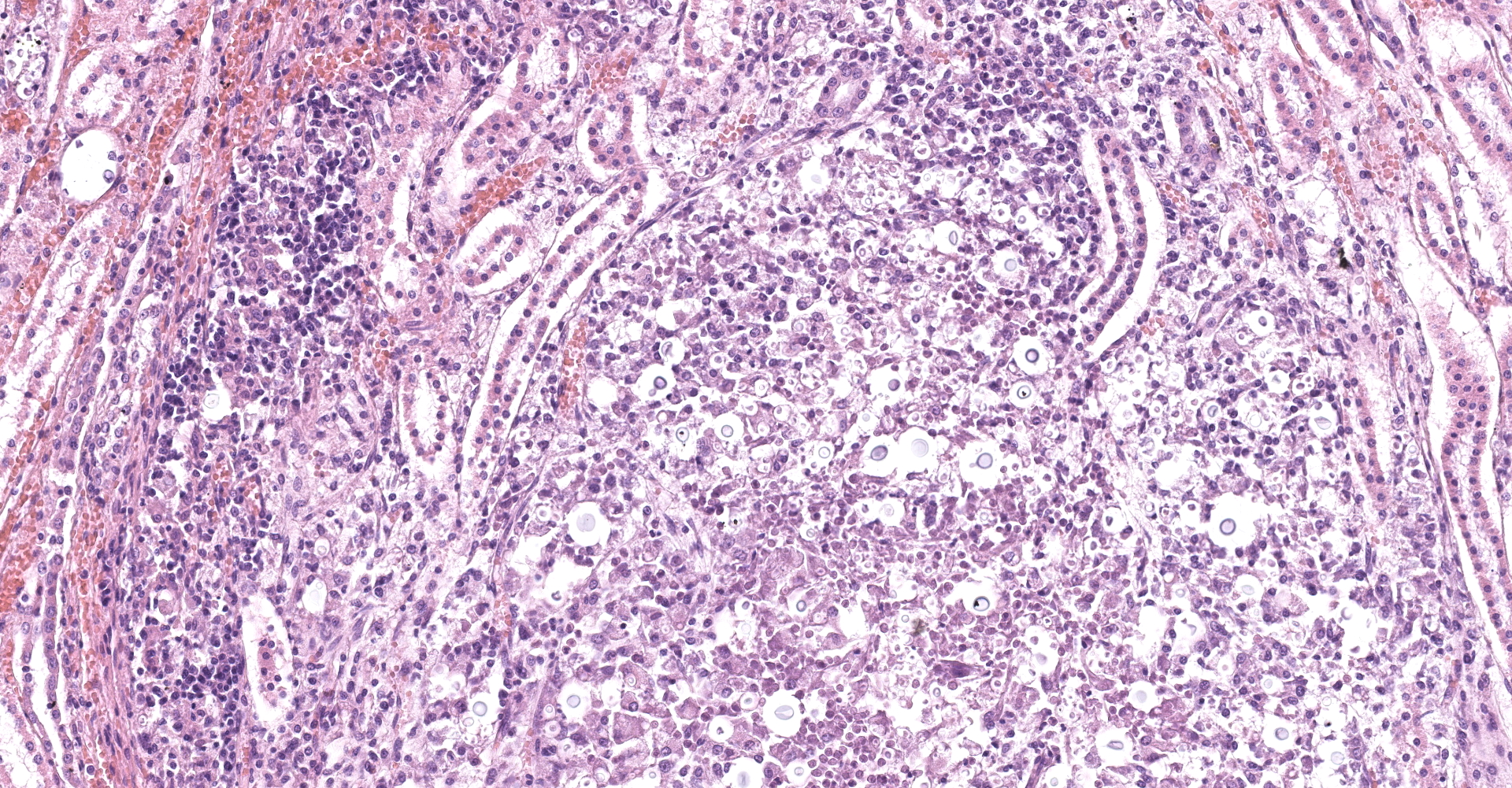
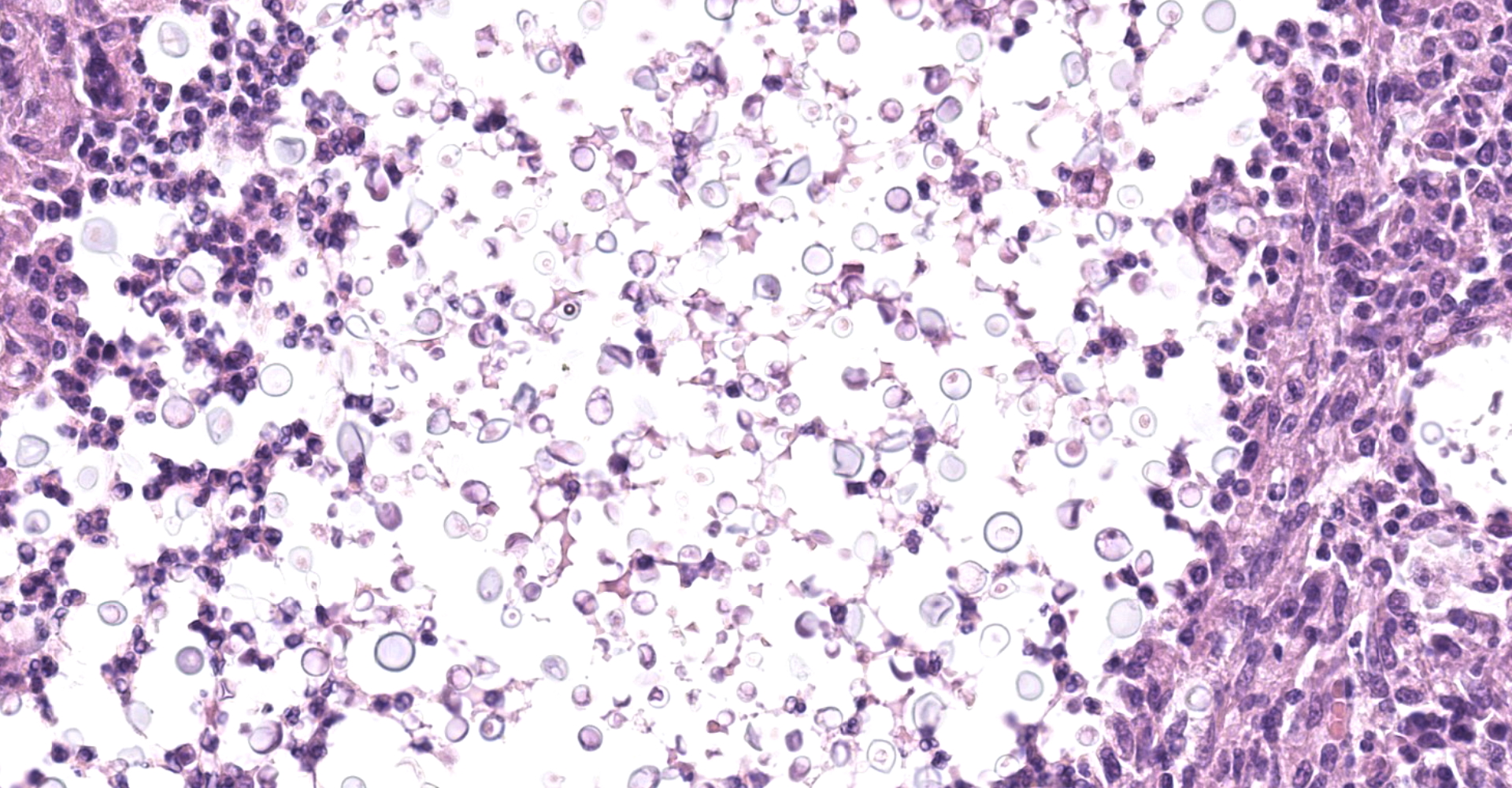
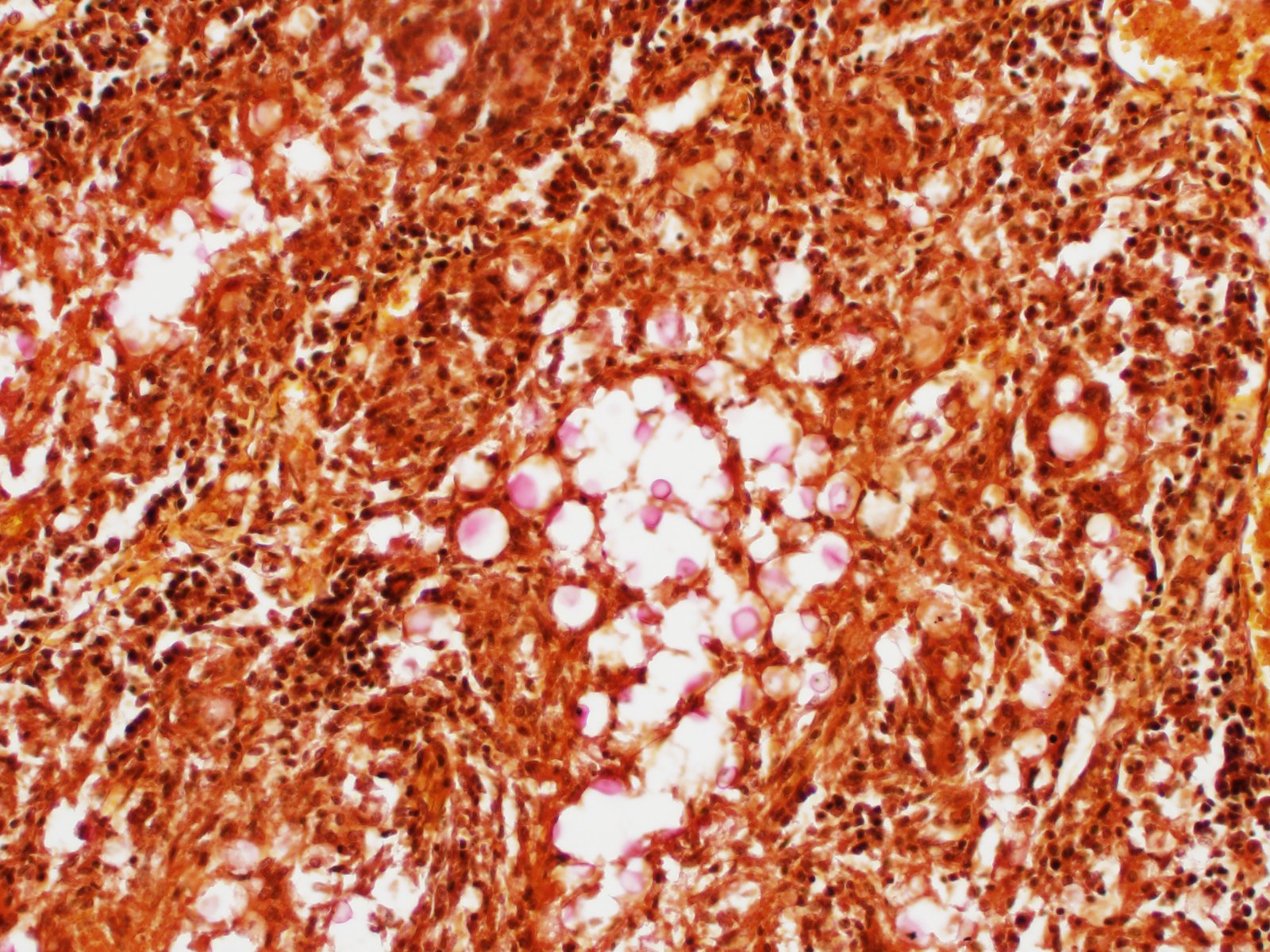
Kidney: Multifocal to coalescing randomly distributed, renal tissue (cortex, medulla, and papilla) and perirenal adipose tissue is infiltrated by a myriad of yeasts admixed with numerous neutrophils, macrophages and a variable number of lymphocytes and plasma cells. The inflammatory cells and yeast along with cellular debris surround the remaining vital tubular epithelial and interstitial cells. The described yeasts are oval to round, 5-10 µm in diameter, with a thin wall (1-2 µm) and are surrounded by clear tick capsule (10-20 µm). Rarely, narrow-based budding of the yeast is visible. Multifocal, predominantly in the areas of pronounced lymphoplasmacytic infiltration, proliferation of fibroblast is present (granulation tissue). Tubular epithelial and glomerular cells multifocal (predominantly in distal tubules) exhibits signs of degeneration and necrosis. Renal tubules and periglomerular spaces are filled with highly proteinaceous fluid. Renal cortex and in to a lesser extent medulla are diffusely congested and edematous with multifocal areas of hemorrhages.
Contributor's Morphologic Diagnoses:
Kidney:
Nephritis, pyogranulomatous and lymphoplasmacytic, multifocal to coalescing,
severe with numerous intralesional yeasts (morphology of the yeasts is
consistent with Cryptococcus spp.).
Contributor's Comment:
The presented slide is sample from a case of disseminated systemic cryptococcosis with macroscopic and histological pyogranuloma-tous inflamation in the kidneys, lungs, liver, pancreas, spleen, heart, intestine, eye, brain, spinal cord and lymph nodes.
Cryptococcus has a worldwide distribution and can affect many animal species and humans. Systemic mycosis is rare and more commonly affects cats than dogs.1,2 Cryptococcosis in dogs and cats is caused by Cryptococcus neoformans and Cryptococcus gattii, which are dimorphic, encapsulated, basidiomycetous fungi.11,12 Cryptococcus neoformans is usual cause of disease in temperate climates and C. gatti was found mainly in tropical climates, but is now considered to have a global distribution. C. neoformans and C. gatti exist in soil, pigeon or other avian guano, and decaying organic matter in a filamentous form known as a teleomorph, with the name Filobasidiella neoformans and F. bacillosporus, respectively; this form of the organism undergoes both sexual as well as asexual reproduction in the environment. Dogs are primarily infected with C. neoformans (except in regions with high endemicity for C. gatti, such as the Pacific Northwest of North America), whereas C. gatti is more frequent in cats.2
Immune suppression, such as from corticosteroid therapy or pre-existing infections, often underlies C. neoformans infections, but C. gatti is a primary pathogen and may infect hosts without known immune deficiencies. Consequently, in cats, the infection frequently occurs in animals that are not immunosuppressed, and feline immunodeficiency virus infection does not worsen the prognosis.1, 2
The main virulence factors of Cryptococcus are the capsule and the production of melanin. The thick capsule, composed of glucuronoxylomannan and other mannose-rich polysaccharides, impairs phagocytosis, activates complement, and may suppress T-cell responses. The role of the capsule in pathogenicity is visible in rare Cryptococcus strains without capsule that provoke severe granulomatous reaction and are minimally pathogenic. The ability to synthesize melanin and enzyme phenoloxidase (laccase) is important for scavenge oxygen radicals produced by macrophages. Cryptococcal yeasts also secrete eicosanoids and mannose protein that modulate immune and inflammatory responses, and production of superoxide dismutase.2 In addition to the fact that in most animals cryptococcosis is more common in immunosuppression, Cryptococcus neoformans and gatti themselves can cause severe immune-suppression.9 The cryptococcal capsular polysaccharide, glucuronoxylomannan effectively inhibits phagocytosis and interferes with migration of leukocytes from the bloodstream into tissues by causing them to shed selectin. It can also deplete complement and directly inhibits T-cell responses.8, 15 There is a shift from a Th1 to a Th2 immune response, the Th1 response being normally required for organism clearance. The cryptococcal urease enzyme was shown to promote accumulation of immature dendritic cells within the lung, and an associated shift in the immune response to a non-protective Th2-cytokine dominated response.8 Immunity to Cryptococcus is dependent on delayed-type hypersensitivity reactions. IFN-γ and other cytokines elicit and activate macrophages and perhaps neutrophils to kill the yeast by using reactive nitrogen and oxygen intermediates. Cytotoxic T-cells directly limit viability or proliferation of this pathogen.2 Recent data indicate that presence of testosterone in men, but not β-estradiol in women, may influence capsule growth and reduce phagocytosis of yeast by macrophages.1 The infection usually occurs by inhalation of basidiospores or desiccated yeast from contaminated dust. Yeasts are replicated into alveoli and are subsequently spread, hematogenously (via macrophages) or locally to other organs (brain, eyes, lymph nodes, skin etc.). Cutaneous cryptococcosis is probably the result of local innoculation and cryptococcal mastitis is the consequence of ascending infection.2, 10
Most infected animals do not develop clinical disease. The disease differs between species; in cats it affects mostly the respiratory tract (rhinitis, pneumonia) wheras in dogs the disease is more disseminated with central nervous system or ocular involvement.2,6 Due to inhalatory route of infection, the lungs and nasal cavity are the most commonly affected organs. Many cases are characterized by chronic nasal disease, with sneezing and serous or mucopurulent discharge. Facial swelling is a common feature of cryptococcal rhinitis of cats. Infection may spread locally from the nasal cavity to involve the skin, oral mucosa, paranasal sinuses, or brain, meninges (through the ethmoid bone, via the cranial nerves or hematogenous spreeding) and eyes.2,11 Less commonly disease has a systemic form with more variable clinical manifestations and this form is more common in dogs. Wider systemic dissemination usually affects the lymph nodes, lung, and distant visceral organs such as liver, spleen, urinary and gastrointestinal tract.
Macroscopic lesions are most commonly in the form of gelatinous masses, granulomas, ulcerating nodules, or depositions of viscous gelatinous exudate on the surfaces of serosal or meningeal membranes. Skin lesions are often nodular and ulcerative. Visceral lesions consist of multifocal discrete white gelatinous lesions. Gross lesions in the brain are often subtle, but may include gelatinous material in meninges and ventricles.2, 3, 5, 7, 11, 13, 14, 16
Histologically, the lesion is remarkable for the large numbers of yeast with abundant nonstaining capsular material that gives a "soap bubble" appearance to the lesion. The inflammatory, mostly granulomatous, reaction is often mild probably due to masking effect of capsule that protect yeasts from phagocytosis. C. neoformans yeast bodies are 4-8 μm diameter, plus a capsule that varies from 1-30 μm. Occasional yeast have single buds that are attached by a thin stalk; this narrow-based budding differentiates Cryptococcus from Blastomyces. Special staining methods, such as periodic acid-Schiff (PAS) and Gomori's methamine silver, demonstrate the organisms, and the capsule can be stained with mucicarmine and alcian blue stain. The inflammatory response is consistent of neutrophils, macrophages, multinucleate giant cells, lymphocytes, plasma cells and eosinophils, which is dependent upon host immune status.2,16
Contributing Institution:
Department of Veterinary Pathology
Faculty of Veterinary Medicine
University of Zagreb
Heinzelova 55
10000 Zagreb
Croatia.
JPC Diagnosis:
Kidney: Nephritis, granulomatous, multifocal, marked, with numerous extracellular and intrahistiocytic yeasts, etiology consistent with Cryptococcus sp.
JPC Comment:
The contributor provides an excellent review of Cryptococcus neoformans and C. gatti, two serious fungal pathogens within phylum Basidiomycota. The range of species susceptible to cryptococcosis is extraordinarily diverse and includes single-celled Acanthamoeba, terrestrial and marine mammal wildlife, small companion animals, horses, production animals, and humans.4
This entity was first described as a human pathogen in 1894 by pathologist Otto Busse and surgeon Abraham Buschke when they isolated a 'Saccharomyces-like' organism from a bone infection in a young woman. In 1901, the organism was renamed Cryptococcus neoformans because it did not produce ascospores, a defining characteristic of the genus Saccharomyces.9
The epidemiology of cryptococcosis in humans has shifted dramatically over the last century. Fewer than 300 cases were reported worldwide during the 1950s. This number dramatically increased in the following years due to the increase in the numbers of patients with AIDS or other states of immune compromise. A 2006 study estimated there were over one million annual cases of cryptococcal meningitis worldwide, predominantly within sub-Saharan Africa and other developing regions where treatment is inhibited by lack of infrastructure and cost. Over half these cases result in patient death, yielding fatalities comparable to both tuberculosis and diarrheal disease in these regions.9
Historically, pathogenic Cryptococcus isolates were treated as a single species, C. neoformans. Heterogeneity between isolates within this species was suspected based on the recognition for four serotypes (A, B, C, and D). This was subsequently confirmed by the discovery of two different teleomorphs, Filobasidiella neoformans (Cryptococcus neoformans) and Filobasidiella bacillospora (Cryptococcus gattii). This division was further verified by whole-genome sequencing. In 2002, serotypes B and C were formally reclassified as C. gatti, while C. neoformans included all serotype A, AD, and D strains. It is possible additional species will be identified given molecular typing methods have demonstrated multiple genetically diverse monophyletic clades within both species.4
As noted by the contributor, Cryptococcus neoformans occurs worldwide and is associated with avian excreta protected by light and is the predominant cause of human and animal infections. This pathogen is often associated with pigeon guano, however, birds are unlikely to be a reservoir given 0 to 2.2% of samples taken from the feet, crop, and cloacal swabs of Colombiforms and water birds in multiple studies were positive for the pathogen. Instead, it is most likely C. neoformans propagates in the nitrogen-rich guano where they grow, rather than being transferred via an avian alimentary tract. In addition, the core body temperature of birds may be as high as 42-44ºC, which inhibits crytptococcal growth and multiplication. Interestingly, C. neoformans can complete its lifecycle, including mating, on pigeon guano whereas C. gatti does not. Cryptococcus gatti has historically been found in tropical and subtropical regions and is associated with various species of trees, notably eucalyptus, in addition to carob and olive trees within the Mediterranean basin.4,9
Given both koalas and C. gatti share an intimate association with a common environmental niche (i.e. eucalyptus trees), it is not surprising the former has a comparatively high, if not the highest prevalence of cryptococcosis in comparison to other species, with approximately 3% of koala necropsies being attributed to the pathogen. Cryptococcosis occurs in both captive and free-ranging koalas, most commonly affecting the upper and/or lower respiratory tract and spreading to regional lymph nodes in 20% of these cases without further tissue involvement. However, approximately a third of all cases in this species are disseminated, often following a longstanding respiratory illness. Neurotropism is common, as in other species, with 32% of cases in koalas having CNS involvement.4
Marine mammals, including delphinids, porpoises, pinnipeds such as harbor seals and sea lions and baleen whales such as the southern right whale are also susceptible hosts for various mycoses including cryptococcosis. Porpoises and dolphins are the most commonly infected marine mammals. These animals are likely at risk for infection when close to land and exposed to infective propagules (likely spores) in effluent and runoff that has washed into the ocean. Those with blow holes are particularly vulnerable, given they inhale an enormous tidal volume with marked inspiratory effort following an explosive expiration, without the benefit of filtration of inspired air via sinonasal protective mechanisms. As a result, large inoculums of inspective spores can be carried deep into the lower respiratory tract.4
Since around 2000, cryptococcosis has emerged as a cause of disease in both marine mammals and humans, particularly in the vicinity of Vancouver Island and the Pacific Northwest of the United States and Canada. Wordwide, C. gatti has been identified in 45/54 cases of symtomatic cryptococcosis, followed by C. neoformans in five animals, and one case of the less pathogenic C. albidus in a stranded California sea lion. The Cryptococcus species was not identified in the remaining three cases. A common finding in all marine mammal cases was tropism for the lungs, lymph nodes, and gastrointestinal tract, with the lung as the logical portal of entry for infection. Similar to other species, extension of the fungus to the brain has also been reported.4
References:
1. Bielska E, May RC. What makes Cryptococcus gattii a pathogen?. FEMS Yeast Res. 2016;16(1):fov106.
2. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier; 2016:582-583.
3. Cazzini P, Camus MS, Garner BC. Pathology in practice. Cryptococcal osteomyelitis in a dog. J Am Vet Med Assoc. 2013;242(8):1079-1081.
4. Danesi P, Falcaro C, Schmertmann LJ, de Miranda LHM, Krockenberger M, Malik R. Cryptococcus in Wildlife and Free-Living Mammals. J Fungi (Basel). 2021;7(1):29.
5. Headley SA, Mota FC, Lindsay S, et al. Cryptococcus neoformans var. grubii-Induced Arthritis with Encephalitic Dissemination in a Dog and Review of Published Literature. Mycopathologia. 2016;181(7-8):595-601.
6. Marcos R, Malhão F, Santos J, Marques S, Thompson G, Santos M. The cryptic Cryptococcus. Vet Clin Pathol. 2016;45(4):532-533.
7. Newman SJ, Langston CE, Scase TJ. Cryptococcal pyelonephritis in a dog. J Am Vet Med Assoc. 2003;222(2):180-174.
8. Osterholzer JJ, Surana R, Milam JE, et al. Cryptococcal urease promotes the accumulation of immature dendritic cells and a non-protective T2 immune response within the lung. Am J Pathol. 2009;174(3):932?43.
9. Srikanta D, Santiago-Tirado FH, Doering TL. Cryptococcus neoformans: historical curiosity to modern pathogen. Yeast.
10. Sykes JE. Immunodeficiencies caused by infectious diseases. Vet Clin North Am Small Anim Pract. 2010;40(3):409-423.
11. Sykes JE, Malik R: Cryptococcosis, In: J. Sykes, C. Greene, eds. Infectious Diseases of the Dog and Cat, 4th ed. Saunders, St. Louis, 2012. 621-634
12. Sykes JE, Sturges BK, Cannon MS, et al. Clinical signs, imaging features, neuropathology, and outcome in cats and dogs with central nervous system crypto- coccosis from California. J Vet Intern Med. 2010; 24: 1427-1438.
13. Trivedi SR, Sykes JE, Cannon MS, et al. Clinical features and epidemiology of cryptococcosis in cats and dogs in California: 93 cases (1988-2010). J Am Vet Med Assoc. 2011;239(3):357-369.
14. Uchiumi K, Stowe DM, DeVanna JC, Willcox JL, Neel JA. Pathology in practice. Cryptococcus sp in a dog. J Am Vet Med Assoc. 2014;245(8):893-895.
15. Yauch LE, Lam JS, Levitz SM. Direct inhibition of T-cell responses by the Cryptococcus capsular polysaccharide glucuronoxylomannan. PLoS Pathog. 2006; 2(11):e120.
16. Zachary JF: Nervous system. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Mosby, Inc.; 2012: 807-808.