WSC 22-23
Conference 23
Case 4:
Signalment:
1-year and 4-month-old, male entire, Lagotto Romagnolo dog (Canis lupus familiaris).
History:
The dog was presented with a 4-month progressive history of intention head tremors, head bobbing, hypermetria in all limbs, indicating a cerebellar disease, which were slowly progressing in severity. The clinical signs started to be present at 4 months of age. Magnetic Resonance Imaging (MRI) was performed and revealed a diffuse cerebellar cortical atrophy, characterized by a moderately and diffusely reduced in size cerebellum, accompanied by prominent cerebellar folia and sulci. Based on the clinical sings and MRI findings, the main clinical differential diagnosis was a neurodegenerative disease, such as altered autophagy and cerebellar storage disease of the Lagotto Romagnolo due to an unidentified mutation, or another form of cerebellar abiotrophy.
The dog was humanely euthanized and submitted for a post-mortem examination (PME).
Gross Pathology:
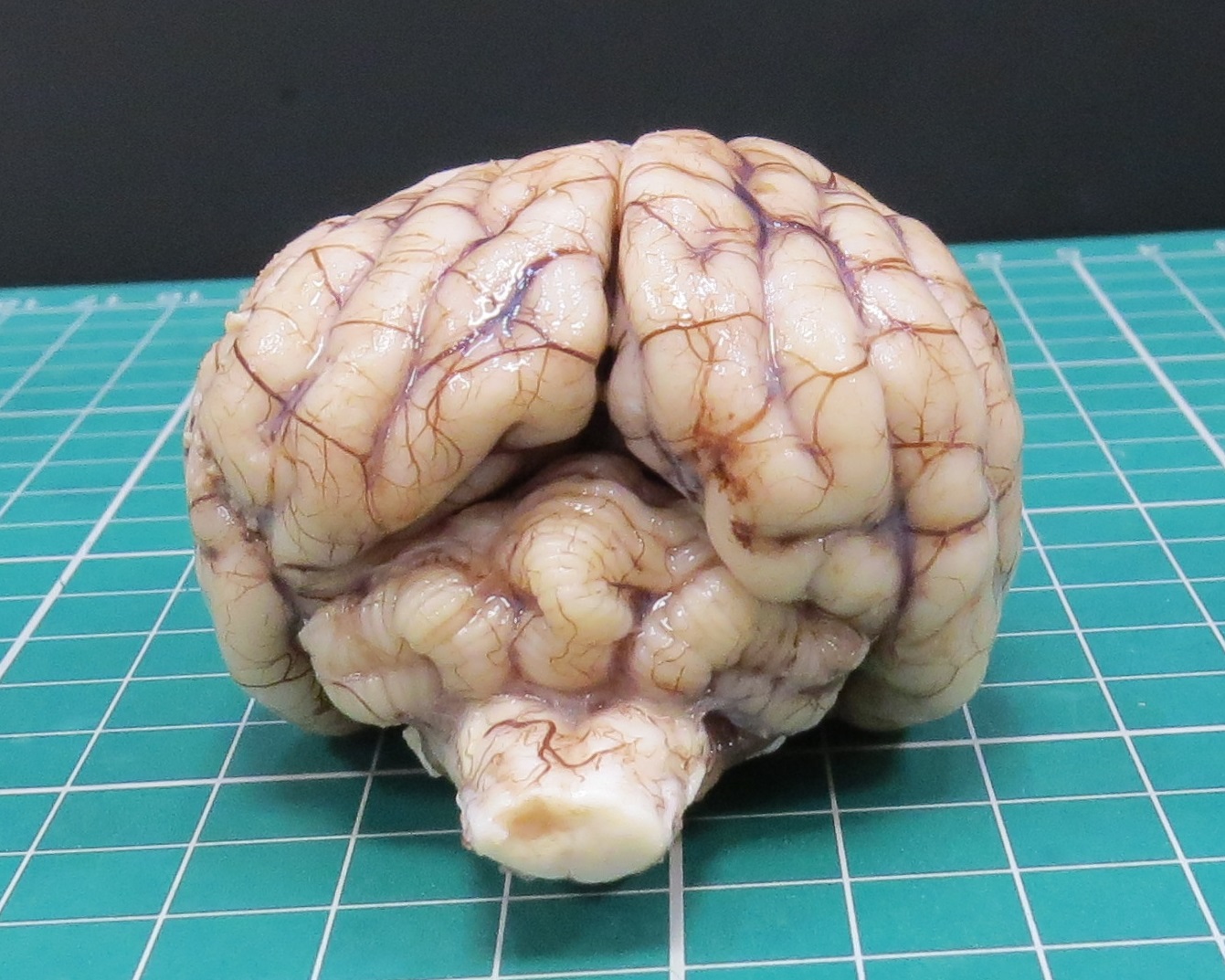
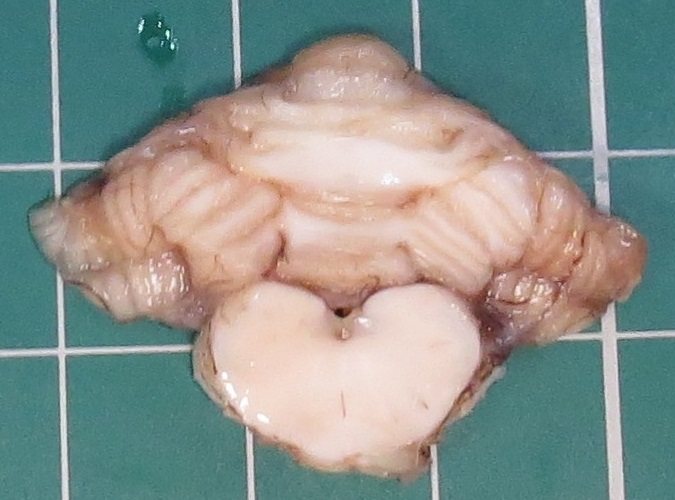
At PME, macroscopic lesions were restricted to the cerebellum, which was diffusely and moderately reduced in size. Upon sectioning of the formalin-fixed cerebellum, the cortical cerebellar folia appeared markedly thinned.
Laboratory Results:
The genetic test LSD for the ATG4D gene mutation, as a specific DNA test for cerebellar storage disease in Lagotto Romagnolo, was negative.
Microscopic Description:
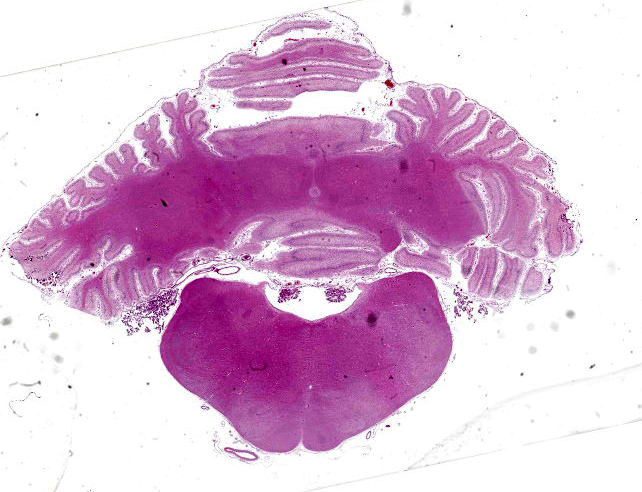
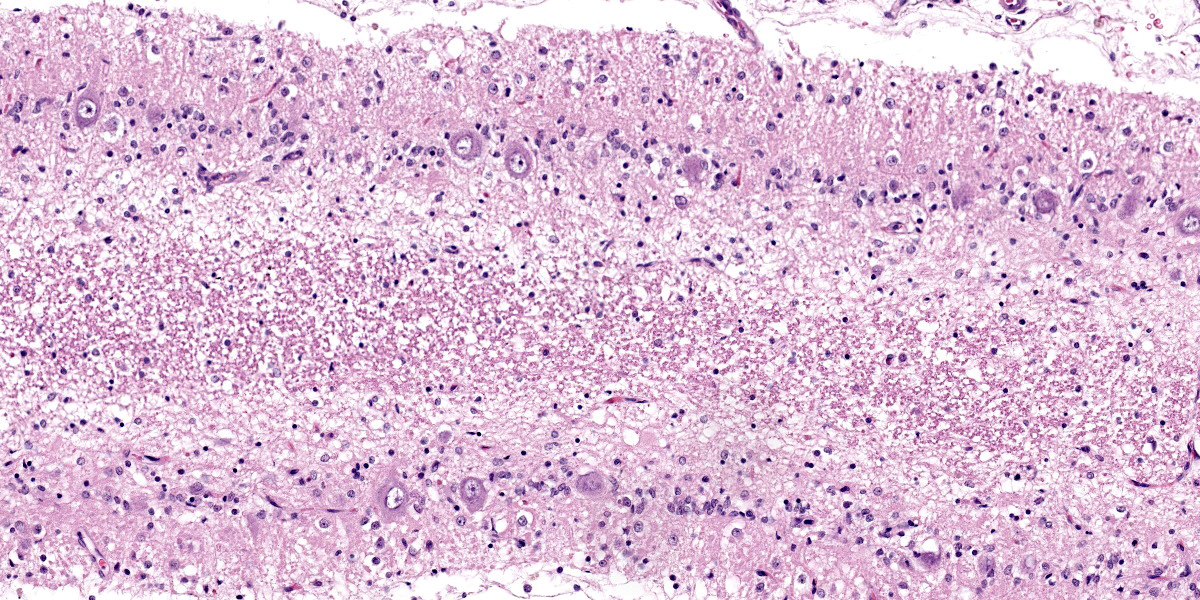
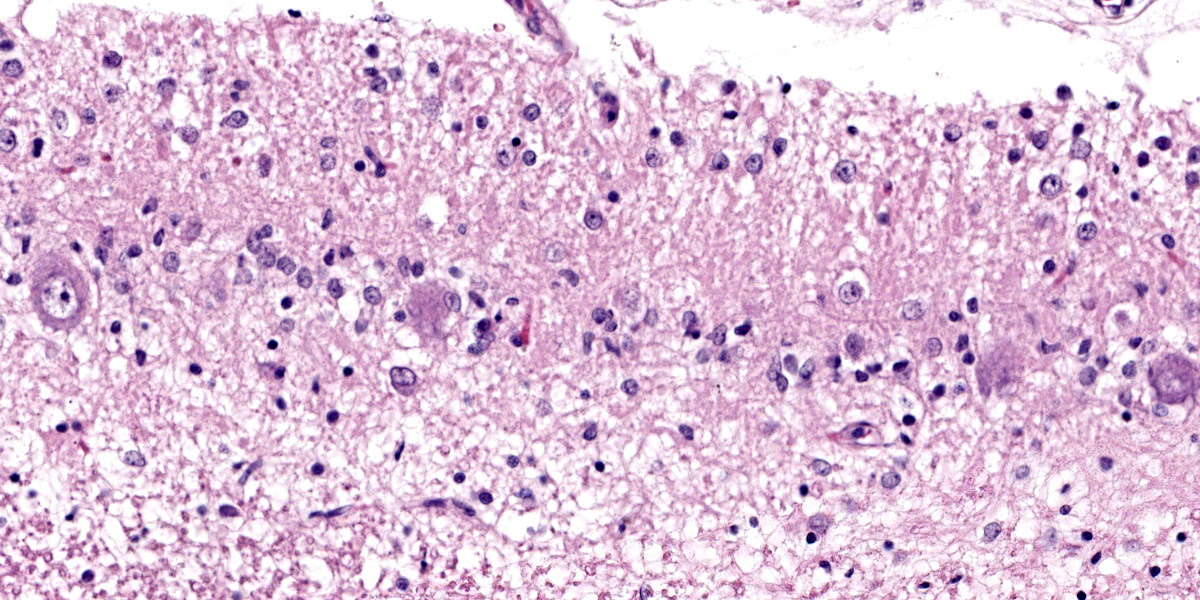
Cerebellum: Diffusely, the cerebellar folia are markedly flattened and there is diffuse, marked thinning and hypocellularity of the granular cell layer with marked loss of granular cells, leading to almost complete absence of this layer accompanied by moderate to marked vacuolation of the neuropil (spongiosis). Rarely, remaining granular cells are either swollen with a vacuolated cytoplasm (degeneration), or shrunken with pyknotic nucleus (necrosis). Glial and microglial cells are diffusely observed replacing the granular layer.
The Purkinje cell layer appears rarely affected with occasional loss of Purkinje cells which are often replaced by large, irregularly round, clear areas (empty baskets). Multifocally, scattered Purkinje cells are either shrunken with angular cellular profile, have a hypereosinophilic cytoplasm and pyknotic nucleus (necrosis) or are rarely swollen with central chromatolysis (degeneration). Rarely, swollen, pale eosinophilic proximal Purkinje cell axons (torpedoes) are observed extending into the granular cell layer.
Diffusely, the molecular layer appears reduced in thickness, but is otherwise unremarkable.
Contributor’s Morphologic Diagnoses:
Cerebellum: Diffuse, severe, granular cell degeneration and loss, with spongiosis, and mild, multifocal Purkinje cell loss
Contributor’s Comment:
Detailed macroscopic examination in a 1-year and 4-month-old Lagotto Romagnolo dog revealed a severe, diffuse, and symmetric cerebellar atrophy, histologically characterized by a diffuse depletion of the cerebellar granular cell layer neurons, with relative sparing of the Purkinje cell layer, compatible with a cerebellar granuloprival degeneration (CGD). CGD is a type of cerebellar cortical degeneration, also termed cerebellar atrophy or abiotrophy.
Cerebellar cortical abiotrophies (CCAs) are a group of rare diseases characterized by premature or accelerated and progressive degeneration and loss of fully developed neurons, secondary to a presumed intrinsic metabolic defect.2 CCA has been described as a hereditary defect in various species, such as various dog breeds7,-9,12,18, Arabian horses14,17, rabbits16, goats10, and rarely in cats2,18.
Animals with CCA are usually neurologically normal at birth and then start to develop progressive signs of cerebellar disease in weeks, months or less commonly years after birth. Histologically, there is a characteristic degeneration and loss of Purkinje cells, which could be accompanied by secondary loss of granule cell neurons, as a retrograde degeneration.4,6
The case presented herein, represents an unusual presentation of cerebellar cortical degeneration/abiotrophy in a Lagotto Romagnolo dog, characterized by marked degeneration and loss of granular cell neurons with relative sparing of Purkinje cells. Based on these histopathological features, this condition has been named cerebellar granuloprival degeneration (CGD).
The main differential diagnosis based on the breed, clinical signs, and MRI findings was altered autophagy and cerebellar storage disease of the Lagotto Romagnolo. This disease is characterized by progressive cerebellar ataxia and cerebellar atrophy as the main MRI finding, as observed in this case. However, the characteristic histopathological changes of the cerebellar storage disease of the Lagotto Romagnolo, consisting of widespread neuronal cytoplasmic vacuolization within both the central and peripheral nervous system, marked progressive Purkinje cell loss accompanied by reduction of granular cell neurons, as well as spheroid formation and cytoplasmic vacuolation in extra-neural tissues (e.g., pancreatic acinar cells, prostate, mammary gland)11, were not observed in the reported case, excluding this disease as a definitive diagnosis.
CGD has been occasionally reported in various dog breeds, including an Australian kelpie and a Labrador retriever, an Italian hound, a Chihuahua, and Border collies, Bavarian mountain and Lagotto Romagnolo dogs.3,7-9,12,15
The etiology and pathogenetic mechanisms leading to CGD are still not completely understood, and, although the development of CCA in some dog breed has been attributed to a genetic abnormality, no specific genetic mutation has been yet identified for canine CGD. Inflammatory and infectious diseases have been considered as differential causes for CGD. In Coton de Tulear dogs, two forms have been recognized: the neonatal cerebellar ataxia (aka Bandera’s neonatal ataxia) caused by a mutation of the GRM1 gene [4], and a suggested immune-mediated form where the destruction of granular cell neurons results from an immune system defect.19
In the presented case, the pathogenetic mechanism leading to CGD was unclear although the histopathological features were not consistent with an inflammatory/infectious etiology. Therefore, a genetic origin was considered more likely. Further studies are needed to elucidate the pathogenesis of this condition in dog breeds.
Contributing Institution:
https://www.nottingham.ac.uk/vet/service-for-business/veterinary-pathology-service/index.aspx
JPC Diagnosis:
Cerebellum: Granule neuron degeneration and loss, diffuse, severe, with mild multifocal Purkinje cell loss.
JPC Comment:
The contributor provides an interesting and uncommon presentation for cerebellar degeneration which was last seen in case 1, conference 12, 2016, in a Coton de Tulear dog.
The moderator explained that granule cells receive most of their stimulation from Purkinje cells, thus are quite susceptible to transsynaptic degeneration during Purkinje cell injury. In the case of granule cell injury, however, Purkinje cells continue to receive stimulation from other neurons (such as the climbing fibers from the olive nucleus), making them more resistant to transsynaptic degeneration. This explains why the secondary Purkinje cell loss was less severe than the primary granule cell loss in this case.
Certain viruses can infect and destroy neuroblast precursors of granule cells in the cerebellum, so an important differential for cerebellar degeneration in young animal to consider is in utero viral infection.20 In addition to granule cell loss, immature Purkinje cells may be fewer in number or malpositioned in the molecular layer, as granule cells to form the scaffolding for migration.13,20 Viruses associated with cerebellar dysplasia include feline and rat parvoviruses; porcine and bovine pestiviruses (classical swine fever virus and bovine viral diarrhea virus), and certain bunyaviruses (Akabane virus, Cache valley virus, and Ainovirus).1,20 Kilham’s rat parvovirus and minute virus of mice (mouse parvovirus) cause cerebellar hypoplasia in rats and mice, respectively, and both of these rodent parvoviruses can also cause cerebellar hypoplasia in experimentally infected Syrian hamsters.1 In pigs, cerebellar hypoplasia can also be induced in utero when sows are treated with certain organophosphates late in gestation.20
Purkinje cells are named after their discoverer, 19th century Czech physiologist Jan Evangelista Purkinje (1787-1869).5 Purkinje conducted extensive research and contributed to the advancement of multiple disciplines, including histology, embryology, and anatomy. The abundance of his research is evidenced in the variety of histoanatomic structures and physiologic phenomena that bear his name. In ocular physiology, Purkinje described dark adaptation, now known as the Purkinje phenomenon, where the perceived intensity of the color red decreases faster than green and blue as light intensity increases. He also described how a bright light in dim surroundings produces four images (Purkinje-Sanson images). These images are generated by the planes of transition as light passes through the eye: the anterior corneal surface, posterior corneal surface, anterior lens surface, and posterior lens surface. With the benefit of improved microscope technology, Purkinje also provide the first description of cells in the cerebellum. Camillo Golgi (with his newly developed silver stain) and Santiago Ramon y Cajal later described structure and processes of these cells, and Cajal recommended the cells be dubbed Purkinje cells. Purkinje’s pursuit of knowledge did not stop with the external world; he also conducted pharmacologic experiments on himself, investigating the effects of agents such as digitalis extract, belladonna, camphor, and turpentine. There is some irony in the long list of discoveries which bear Purkinje’s name, as Purkinje himself stated, “Science is not about names but discoveries.”5
References:
- Barthold SW, Griffey SM, Percy DH. Path,ology of Laboratory Rodents and Rabbits. Ames, IO: John Wiley & Sons, Inc. 2016; 18, 124, 175.
- Biolatti C, Gianella P, Capucchio MT, et al. Late onset and rapid progression of cerebellar abiotrophy in a domestic shorthair cat. J Small Anim Pract. 2010;51:123–126.
- Cantile C, Salvadori C, Modenato M, et al. Cerebellar Granuloprival Degeneration in an Italian Hound. J Vet Med. A 49, 523–525 (2002).
- Cantile C, Youssef S. Nervous system. Maxie MG ed. In: Jubb Kennedy and Palmer’s Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia, PA: Elsevier Saunders; 2016:276, 318-320.
- Cavero I, Guillon JM, Holzgrefe HH. Reminiscing about Jan Evangelista Purkinje: a pioneer of modern experimental physiology.
- Coates JR, O’Brien DP, Kline KL, et al. Neonatal Cerebellar Ataxia in Coton de Tulear Dogs. J Vet Intern Med. 2002;16:680–689.
- Flegel T, Matiasek K, Henke D, et al. Cerebellar cortical degeneration with selective granule cell loss in Bavarian mountain dogs. J Small Anim Pract. 2007;48, 462–465.
- Huska J, Gaitero L, Heindrich N Snyman, et al. Cerebellar granuloprival degeneration in an Australian kelpie and a Labrador retriever dog. Can Vet J. 2013;54:55–60.
- Jokinen TS, Rusbridge C, Steffen F, et al. Cerebellar cortical abiotrophy in Lagotto Romagnolo dogs. J Small Anim Pract. 2007;48:470–473.
- Koehler JW, Newcomer BW, Holland M, et al. A Novel Inherited Cerebellar Abiotrophy in a Cohort of Related Goats. J Comp Path. 2015;153:135-139.
- Kyöstilä K, Syrjä P, Jagannathan V, et al. A Missense Change in the ATG4D Gene Links Aberrant Autophagy to a Neurodegenerative Vacuolar Storage Disease. PLoS Genet. 2015;11(4): e1005169.
- López Betran M, Mascort J, Pumarola M, et al. Cerebellar granuloprival and trans-synaptic degeneration in a Chihuahua. Vet Rec Case Rep. 2020;8:e000873.
- Miller AD, Zachary JF. Nervous System. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th ed. Saint Louis, MO: Elsevier; 2022: 964.
- Sabada SA, Madariaga GJ, Cobi Botto CM, et al. First report of cerebellar abiotrophy in an Arabian foal from Argentina. Open Vet J. 2016; 6(3): 259–262.
- Sandy JR, Slocombe RF, Mitten RW, et al. Cerebellar Abiotrophy in a Family of Border Collie Dogs. Vet Pathol. 2002;39:736–738.
- Sato J, Sasaki S, Yamada N, et al. Hereditary Cerebellar Degenerative Disease (Cerebellar Cortical Abiotrophy) in Rabbits. Vet Pathol. 2012;49(4):621-8.
- Scott EY, Penedo MCT, Murray JD, et al. Defining Trends in Global Gene Expression in Arabian Horses with Cerebellar Abiotrophy. Cerebellum. 2017;16(2):462-472.
- Taniyama H, Takayanagi S, Izumisawa Y, et al. Cerebellar Cortical Atrophy in a Kitten. Vet Pathol. 1994;31:710-713.
- Tipold A, Fatzer R, Jaggy A, et al. Presumed immune-mediated cerebellar granular degeneration in the Coton de Tuléar breed. J Neuroimmunol. 2000;10(1-2):130-133.
- Vandevelde M, et al. Veterinary Neuropathology. Ames, IA: Wiley-Blackwell; 2012: 96-98.