Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 6
28 August, 2019
Dr.
Jeff Wolf, DVM
Senior Pathologist
Chief Scientific Officer/Pathology Manager
EPL, Inc.
Sterling, VA
CASE III: AFRIMS Case 1 (JPC 4113195).
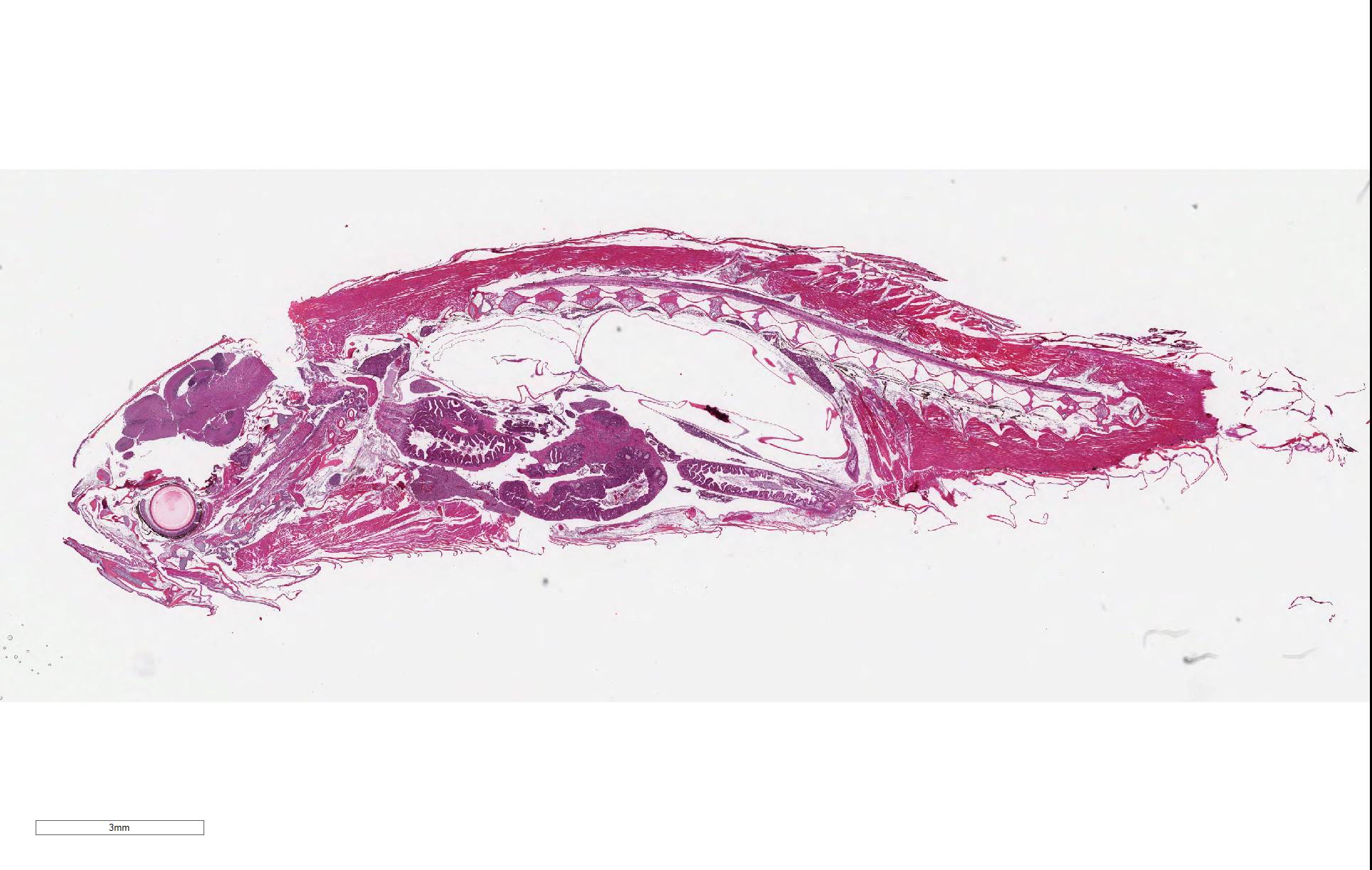
Signalment: Adult, male zebrafish (Danio rerio)
History: This animal was one of four maintained in a private aquarium and was >1 year old. The animal progressively (1 month) was observed to have developed a swollen abdomen and then became anorexic for 1 week. The last two days prior to euthanasia, the animal lost buoyancy control and swam irregularly within the water column. Necropsy did not reveal any abnormalities. All other animals in the tank were within normal limits.
Gross Pathology: Necropsy did not reveal any abnormalities.
Laboratory results: N/A
Microscopic Description:
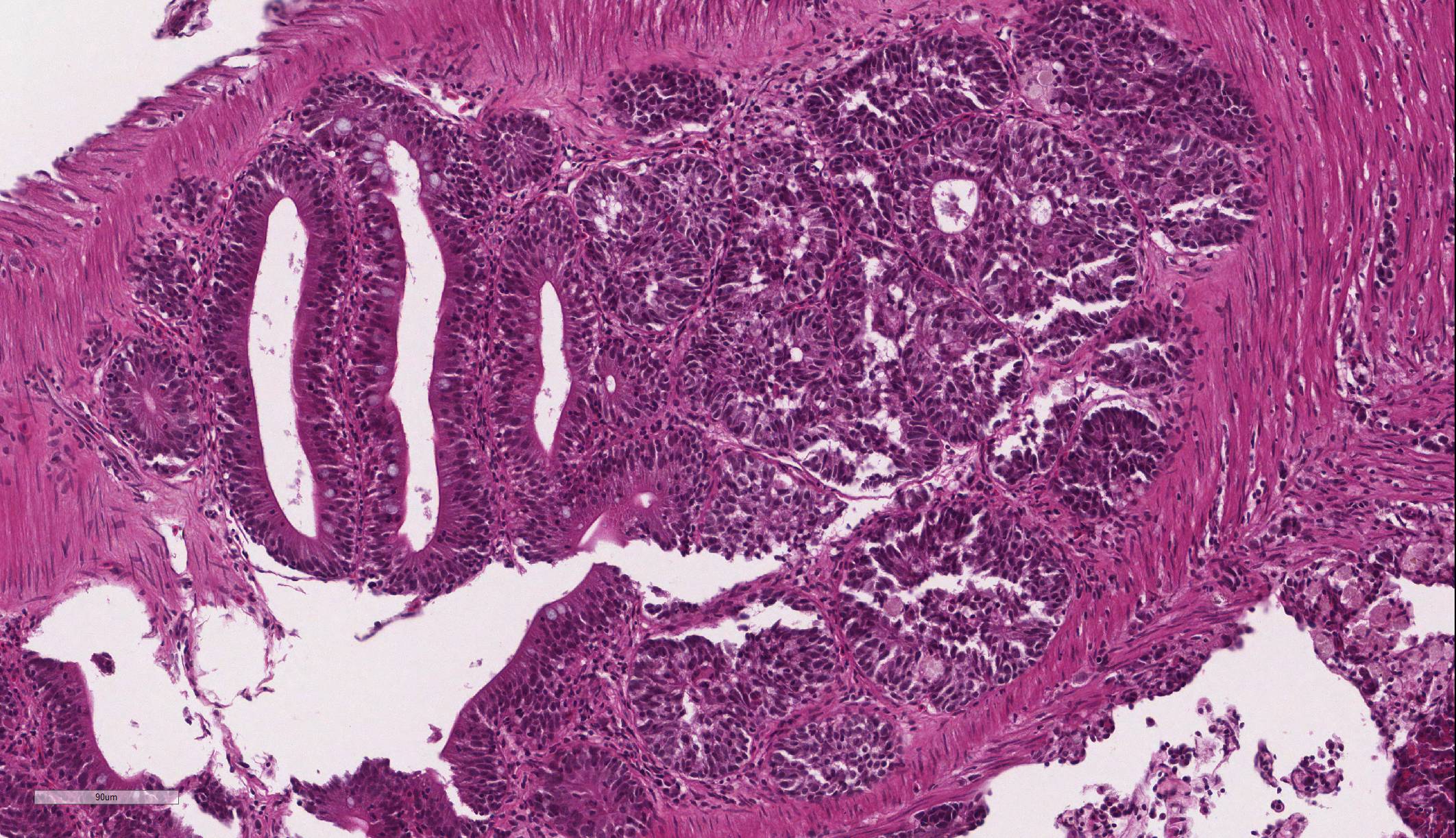
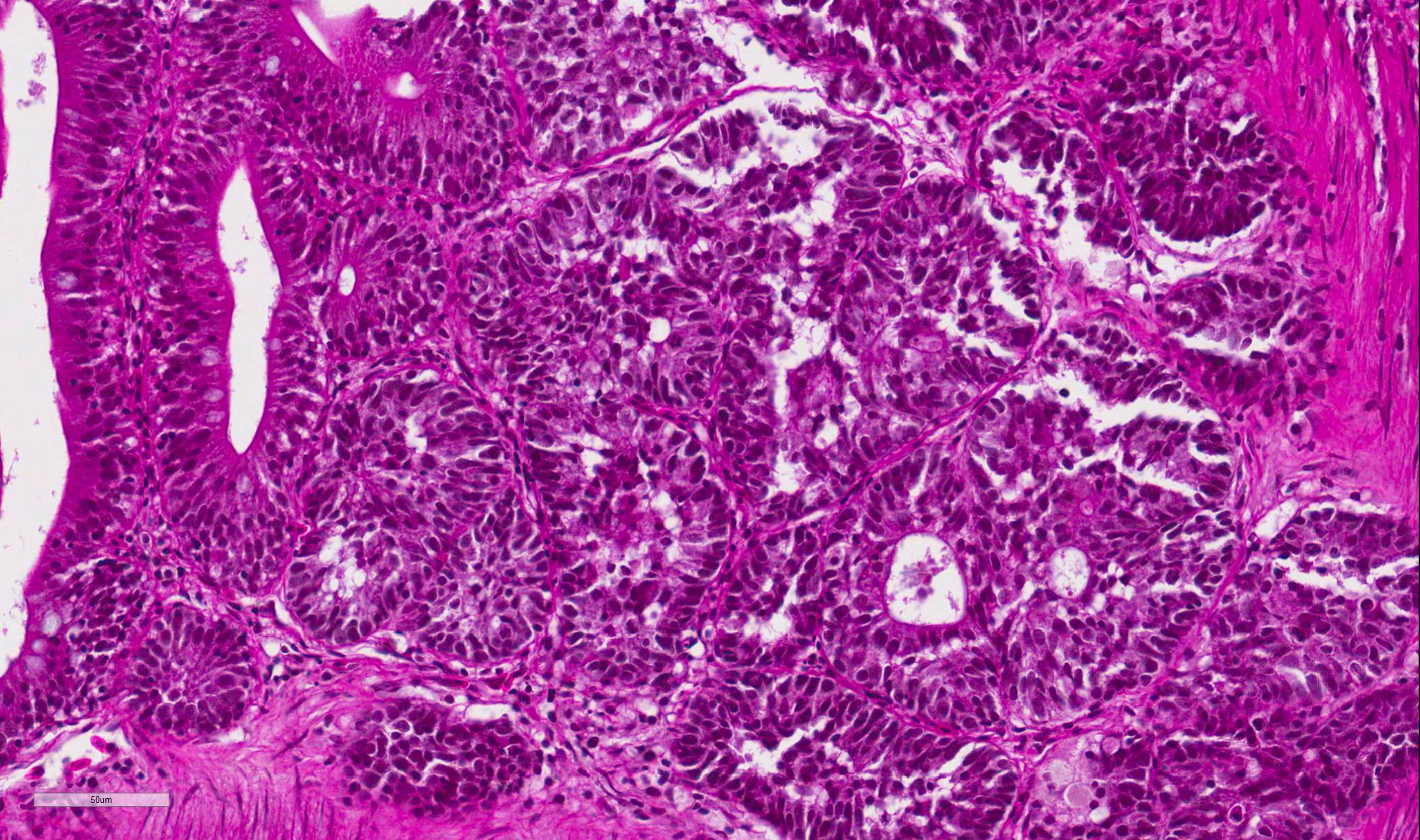
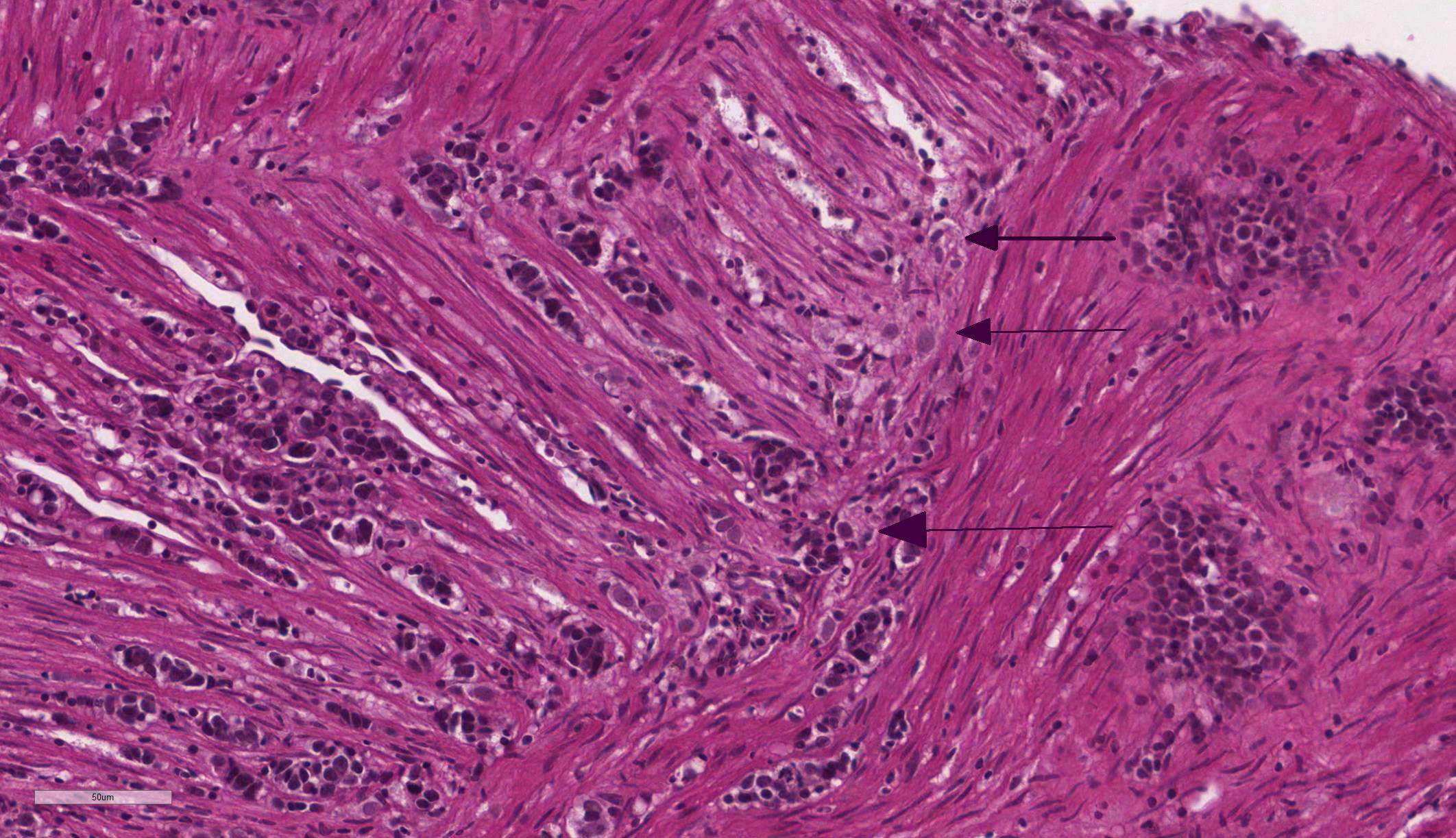
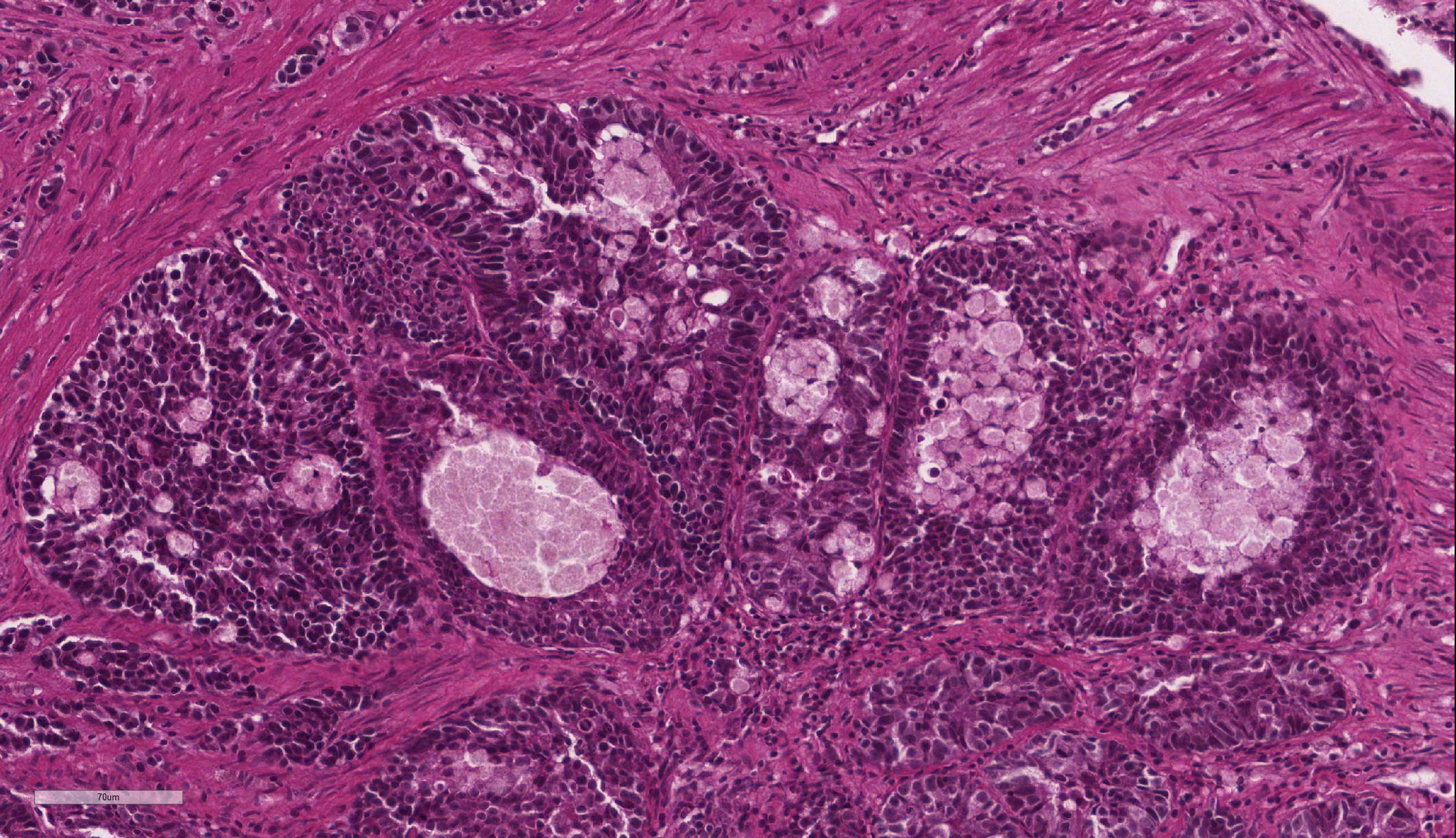
Intestine: Multifocal to coalescing, markedly expanding and effacing the intestinal mucosa and submucosa and extending multifocally into the muscularis is a densely cellular, unencapsulated, poorly circumscribed neoplasm composed of polygonal cells arranged in abortive tubules in which the cells pile up and frequently have lost polarity, as well as disorganized islands and trabeculae on a fine fibrovascular stroma. Neoplastic cells have variably distinct cell borders, small to moderate amount of basophilic granular cytoplasm, oval to elongate nuclei with dense, finely stippled chromatin and occasionally 1 distinct nucleolus. Mitoses are infrequent at approximately 1 per 3 high powered fields observed. There is moderate anisocytosis and anisokaryosis, and often neoplastic cells within tubules appear to undergo maturation to mucoid cells which are characterized by large polygonal cells with abundant microvacuolated eosinophilic cytoplasm and small peripheralized nuclei with dense chromatin and no observable nucleoli. No mitoses were observed in this population. Neoplastic tubules are occasionally either occluded by the proliferating cells or are rarely ectatic and contain eosinophilic cellular and karyorrhectic debris, mucous, sloughed cells, scattered granulocytes and lymphocytes. Multifocally, extending transmurally and occasionally disrupting the serosal surface, the myocytes within the affected muscularis are separated, surrounded and replaced by small islands of neoplastic cells, eosinophilic amorphous material (edema), streams of lymphocytes and granulocytes. In these areas, myocytes are vacuolated and swollen (degenerate). Adjacent mucosa, unaffected by the neoplastic process, is frequently hyperplastic with epithelial piling and formation of papillary fronds which occasionally occlude the crypts. Focally, within a pancreatic vessel there is an organizing fibrin thrombus; however neoplastic cells are not observed within vessels or lymphatics. Occasionally rhabdomyocytes within the body wall are degenerate, necrotic or atrophic and separated by the previously described edema and inflammatory cells.
Liver; kidney; rectum; branchial arch; gill; thyroid gland; esophagus; oral cavity; brain; eye; spinal cord; vertebral column; air sac: No significant findings.
Contributor Morphologic Diagnosis:
Intestine: Adenocarcinoma
Contributor Comment: Fish have been and continue to be used extensively as comparative animal models for human neoplastic disease, and the zebrafish is by leaps and bounds the primary species employed. There are many reasons why zebrafish have made such headway into the fields of cancer research, and while the adult animal may appear to share little with primates, there are a wide range of evolutionarily preserved signaling pathways, translational regulation of cellular division and molecular markers of neoplasia which make fish extremely relevant to comparative pathology studies.5,9,10,11 Furthermore, the embryology of fish and humans share significant commonality and as such present a valuable resource for study of the development of human disease, especially with embryonal tumors.5,10 A recent and extremely exciting development of a transparent adult zebrafish allows for in vivo assessment of neoplastic metastatic behavior using labeled neoplastic cells.13
In addition to spontaneous tumors, neoplastic disease has been induced in fish and in particular in zebrafish using a variety of methods. genetic manipulation, xenotransplantation, chemical carcinogenesis, forward and reverse genetic screens and radiation induction of neoplasia have all been described.5,9,10,11,12 Mutant zebrafish lines have been created which are highly susceptible to the development of tumors, especially, but not limited to, those which are rare in other vertebrate species such as chordomas, pineocytomas, hepatoblastomas, ocular medulloepitheliomas and olfactory esthesioneuroepitheliomas.12
Zebrafish lack a stomach, and as such, the esophagus connects directly to the intestine. At a cellular level, with the exception of Paneth cells, the zebrafish intestine is composed of all of the same cell types which make up the intestinal tract in mammalian species. The intestine is a contiguous simple tube which has morphological differences at the cellular level, primarily associated with the cellular populations and changes in the epithelium along its length, but that does not have macroscopic features which allow differentiation of large and small sections. 6,10
Intestinal
adenocarcinoma in zebrafish has been reported to occur with some frequency as a
spontaneous neoplasm,2,3,7,10,11 as well as occurring in the
genetically modified adenomatous polyposis coli-deficient zebrafish (APC)
model.2,3,10 Additional reports of experimentally induced intestinal
tumors have been associated with other genetically modified zebrafish lines,
animals exposed to carcinogens and transgenic as well as in association with
the nematode parasite, Pseudocapillaria tomentosa.4,10
A large study was conducted at Oregon State University examining submissions to the Zebrafish International Resource Center (ZINC) over a 10-year period. Approximately 2% of the cases submitted during that period were diagnosed with intestinal lesions, and of those, 113 tumors were diagnosed, with greater than 50% receiving diagnoses of adenocarcinomas.7
The histopathological criteria for diagnosis of the various intestinal lesions, which were described in the evaluation, are abstracted below as Table 1 adapted from Paquette et al.âs retrospective study on intestinal neoplasia in zebrafish.7
Table 1. Defining Histological Signs of Intestine Presentations as Observed within Zebrafish Submitted to the Zebrafish International Resource Center Diagnostic Service 2000-20127
|
Intestinal Presentation |
Defining Signs |
|
Normal Intestine |
One cell thick layer of columnar epithelial cells lining mucosal folds with basally-oriented oval nuclei; mucosal folds become progressively shorter caudally, causing âvilliâ (the normal undulating structure of the intestinal wall appears villous, but lacks the true anatomic characteristics of villi) to appear shorter as the intestine approaches the excretory vent (anus); lamina propria, but no submucosa; inner circular and outer longitudinal smooth muscle layers invest the intestine throughout its length. Mucosal mucus (goblet) cells can be observed and increase in number distally.
|
|
Hyperplastic Intestine |
Multilayered and increased numbers of epithelial cells, especially within basilar mucosal folds; âpiling-upâ of mucosal epithelial cells; nuclear pseudostratification; enhanced nuclear basophilia; pseudocrypt formation resulting from increased mucosal folding; anisokaryosis frequently observed and increased mitotic figures.
|
|
Dysplastic Intestine |
Features of hyperplastic intestine in addition to increased nuclear and cellular pleomorphism, and occasionally aberrant mitotic figures, the loss of nuclear polarity and disorganization or absence of pre-existing histoanatomic architecture |
|
Intestinal adenocarcinoma |
Features of dysplastic intestine plus formation of disorganized pseudocrypts with invasion deep into the lamina propria and frequently through the basement membrane into the underlying muscularis layers; bizarre mitotic figures; neoplastic epithelial cells are pleomorphic and may be columnar, cuboidal or attenuated; hyperchromatic nuclei; annular strictures and fibroplasia frequently accompany tumorigenesis; pseudocrypts formed by the folding of neoplastic mucosal epithelium often resembled pseudoacinar structures that contained intraluminal sloughed rafts of necrotic neoplastic cells |
|
Intestinal small cell carcinoma |
Sheets and nests of round, polygonal or fusiform cells with minimal cytoplasm; hyperchromatic nuclei with granular chromatin and inconspicuous nucleoli; extensive fibroplasia; tumor cells occasionally formed an insular or organoid pattern characteristic of neuroendocrine tumors.
|
|
Intestinal tubular/tubulovillous adenoma |
Focal adenomatous polypoid structures with clusters resembling mammalian glandular colonic crypts. The pseudocrypts often are lined by hyperplastic mucosal epithelium where the cells are crowded and have hyperchromatic nuclei. Increased mitotic figures are observed. Tubulovillous adenoma is essentially similar to tubular adenoma with a combination of both villous and pseudocrypt structures
|
The neoplasm in this case was diagnosed as part of the histopathological evaluation of the sampled tissues, rather than as a post-mortem necropsy finding. The clinical symptomology prior to euthanasia is consistent with the final diagnosis, however is relatively non-specific for any abdominal neoplasm as well as a wide range of other pathological processes. The histopathological features of this tumor are consistent with a diagnosis of intestinal adenocarcinoma.
Contributing Institution:
Armed Forces Research Institute of Medical Sciences (AFRIMS). http://afrims.amedd.army.mil/usamd-afrims.html
JPC Diagnosis: 1. Intestine:
Adenocarcinoma.
2. Pancreas: Intestinal adenocarcinoma, metastatic.
JPC Comment: The contributor has provided an excellent and comprehensive review of this neoplasm and its importance in a common laboratory species. Realizing the complexity of diagnosis of these tumors based solely on their morphologic appearance,7 Paquette et al. in 20158 published a study detailing the immunohistochemical profile of these tumors, identifying them as neoplasms of epithelial origin (rather than their primary differential of tumors of neuroendocrine origin.) These neoplasms stained positive for AE1/AE3 (cytokeratin), while no tumors exhibited any immunopositivity for neuroendocrine markers (chromagranin A or S-100.)8
An interesting study by the Oregon State group in 2018 strongly suggests that this particular entity is transmissible.1 The condition has been identified across zebrafish of multiple genetic backgrounds, suggesting genetics did not play a strong role. Feeding diets of fish with a high prevalence to fish with a low prevalence did not increase the frequency of the finding, suggesting diet was not a factor. Connecting tanks of fish with high prevalence to those of low prevalence in the same recirculating system did not result in a spike in tumors. Following a cohabitation protocol with fish with a known high incidence of the tumor, sampling of the biome of these fish with high-throughput 16S rRNA sequencing was performed, which indicated a high rate of infection in animals with tumors with a yet unidentified species of Mycoplasma, presumably spread to naive fish via a fecal-oral route. The authors do admit that a) other agents, potentially oncogenic viruses, may still be causative or play a role in causation, and b) the Mycoplasma infection may be a result of the pathological change rather than its cause. They recommend managing fish with the condition as having a potentially transmissible condition.1
References:
1. Burns AR, Watral V, Sichel S,
Spagnoli S, Banse AV, Mittge E, Sharpton TJ, Gullemin K, Kent ML. Transmission
of a common intestinal neoplasm in zebrafish by co-habitation. J Fish Dis
2018 41(4):569-579.
2. Feitsma H, Cuppen E. Zebrafish as a cancer model. Mol Cancer Res.
2008; 6:685-694. Shive HR. Zebrafish models for human cancer. Vet
Pathol. 2013; 50:468-482.
3. Haramis AP, Hurlstone A, van der Velden Y, et al. Adenomatous polyposis coli-deficient zebrafish are susceptible to digestive tract neoplasia. EMBO Rep. 2006;7:444â449.
4. Kent ML, Bishop-Stewart JK, Matthews JL, et al. Pseudocapillaria
tomentosa, a nematode pathogen, and associated neoplasms of zebrafish (Danio rerio) kept in research colonies. Comp Med. 2002;52:354â358.
5. Langheinrich U, Hennen E, Stott G, Vacun G. Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Current Biol. 2002; 12:2023-2028.
6. Menke AL, Spitsbergen JM, Wolterbeek APM, et al. Normal anatomy and
histology of the adult zebrafish. Tox Pathol. 2011;39:759-775.
7. Paquette CE, Kent ML, Buchner C, et al. A retrospective study of the prevalence and classification of intestinal neoplasia in zebrafish (Danio rerio). Zebrafish. 2013; 10:1-9.
8. Paquette,
CE, Kent ML, Peterson TS, Wang R, Roderick HD, Lohr CV. Immunohistochemical
characterization of intestinal neoplasia in zebrafish indicated epithelial
origin. Dis Aquat Org 2015; 116(3): 191-197.
9. Setlow RB, Woodhead AD, Grist E. Animal model for ultraviolet radiation induced melanoma: Platyfish-swordtail hybrid. Proc Natl Acad Sci USA. 1989;86:8922-8926.
10. Shive HR. Zebrafish models for human cancer. Vet Pathol. 2013; 50:468-482.
11. Shive HR, West RR, Embree LJ et al. brca2 and tp53 collaborate in tumorigenesis in zebrafish. PLoS ONE. 9(1): e87177.doi:10.1371/journal.pone.0087177.
11. Spitsbergen
JM, Kent ML. The state of the art of the zebrafish model for toxicology and
toxicologic pathology research - Advantages and current limitations. Tox
Pathol. 2003;3(Suppl.):62-87.
13. White RM, Sessa A, Burke C, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008; 2:183â189.