Signalment:
Gross Description:
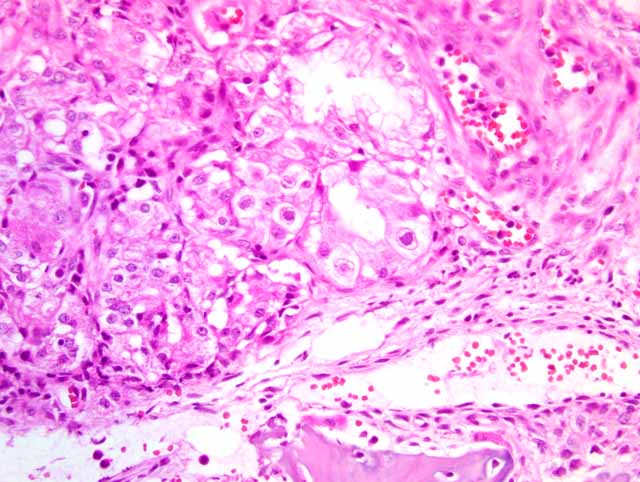
Histopathologic Description:
There is a large focally extensive necrotic area (not included in every slide), characterized by a large amount of eosinophilic karyorrhectic and pyknotic cell debris, and bordered by an abundant amount of small and medium caliber vessels surrounded by reactive fibroblasts and collagen (granulation tissue).
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Infection can be diagnosed by the presence of characteristic large, basophilic, intranuclear inclusion bodies in cytomegalic cells of the nasal glandular epithelium. Such inclusion bodies and mononuclear cellular infiltrations can also be detected in the tubular epithelia of the kidneys, as well as the epithelia of the salivary and tear glands. Other, less frequent, lesions include interstitial pneumonia and lymphocytic perivasculitis in the brain. Predisposing factors of the infection are not fully known. A low level of immunity within the herd, e.g., in newly established herds, and immunosuppressive effects may play a role. Seroconversion due to PCMV is probably much more frequent than clinical disease.(1) In our case, sows in the herd had problems with insufficient lactation.
Herpesviridae
| Alphaherpesvirinae: focal lesions in skin and mucosa of resp. and genital tract; abortion; neonates: necrosis in multiple organs, latency in nerves | Equine herpesvirus 1: Equine herpesviral abortion, rhinopneumonitis, neurologic disease | horse |
| Equine herpesvirus 3: Equine coital exanthema | horse | |
| Equine herpesvirus 4: Equine rhinopneumonitis | horse | |
| BHV-1: infectious bovine rhinotrcheitis/infectious pustular vulvovaginitis/infectious balanoposthitis | cattle | |
| BHV-2: bovine mammilitis virus (bovine herpes mammilitis) | cattle | |
| BHV-5: bovine herpesvirus encephalitis | cattle | |
| SHV-1: Aujezky-�s disease, Pseudorabies | pig > others | |
| Canine herpesvirus 1: | dog | |
| Feline herpesvirus 1: upper respiratory tract disease (rhinotracheitis) and conjunctivitis (ulcers) | cats | |
| Feline herpesvirus 1: feline herpesvirus ulcerative dermatitis | cats | |
| Gallid herpesvirus-1: Infectious laryngotracheitis (ILT) | chicken | |
| Gallid herpesvirus-2: Marek-�s disease | chicken | |
| Psittacine herpesvirus: Pacheco's disease | psittacines | |
| Anatid herpesvirus-1: Duck plaque/Duck virus enteritis | ducks, geese, swan | |
| Simplexvirus: HSV-1, HSV-2, HBV, BHV-2 | ||
| Herpesvirus simplex, type 1/type 2 | human & nonhuman primates | |
| Herpesvirus simiae/Herpes B/Cercopithecine HV | rhesus macaques | |
| Simian varicella virus | macaques, AGM, Patas monkeys | |
| Betaherpesvirinae: no cell lysis, karyomegaly, latency in secretory glands, lymphoreticular organs, kidney | HHV-5, HHV-6, MCMV-1 | humans |
| Porcine herpesvirus 2: porcine cytomegalovirus disease/Inclusion body rhinitis | porcine | |
| Cytomegalovirus | humans & nonhuman primates | |
| Gammaherpesvirinae: primates: lymphoproliferative disease, latency in lymphoid tissue | EHV-2 | |
| EHV-5 | ||
| BHV-4: bovine herpes mammary pustular dermatitis | cattle | |
| OHV-2/AHV-1: malignant catarrhal fever | various ruminants | |
| Epstein-Barr virus (lymphocryptovirus-gamma 1) | primates | |
| Kaposi-sarcoma-associated herpesvirus/human herpesvirus-8 (KSHV/HHV8) (Rhadinovirus-gamma 2) | primates | |
| Deltaherpesvirinae | Anatid herpesvirus-1: duck plague | |
| SHV-2: Eischlussk+�-�rperchenkrankheit | pig | |
| Karpfenpocken | fish | |
| Uncharacterized viruses | Koi-herpesvirus (KHV, carp nephritis and gill necrosis virus, CNGV, Cyprinid-Herpesvirus-3, CyHv-3) | fish |
JPC Diagnosis:
1. Nasal turbinates: Rhinitis, necro-ulcerative, subacute, diffuse, moderate, with glandular epithelial eosinophilic intranuclear inclusions
2. Haired skin and bone: Necrosis, focally extensive, with granulation tissue, fibrosis, osteonecrosis and osteolysis
Conference Comment:
Both Bordetella bronchiseptica and Pasteurella multocida produce their own toxins, and the severity of disease is dependent on the amount of toxin absorbed. One major difference between these two organisms is the age of affected pigs. PAR can affect pigs older than 3 months of age, whereas NPAR normally only affects pigs up to 6 weeks of age.(2)
Gross lesions in PAR are restricted to the nasal cavity and adjacent bone with the ventral scrolls of the nasal turbinates most often suffering the worst lesions. Histologically, the pathognomonic lesion of PAR is replacement of the bony plates of the ventral conchae with fibrous tissue. Metaplasia of adjacent respiratory epithelium is also common.(2)
References:
2. De Jong MF:Progressive and nonprogressive atrophic rhinitis. In: Diseases of Swine, ed. Straw BE, Zimmerman JJ, DAllaire S, Taylor DJ, 9th ed., pp.577-602. Blackwell Publishing, 2006