Signalment:
10-year-old, female, (
Lama pacos) AlpacaThe animal was presented to the clinicians of
the Institute of Farm Animals of the University of Zurich
because of cachexia and weakness although it seemed
to be eating normally. Clinical findings were intensified
breathing, weakness and loss of gastrointestinal motility.
The sonografic picture of the liver showed multifocal,
echogenic, round to sickle-shaped unencapsulated
structures of about 1 to 3 cm in diameter.
Gross Description:
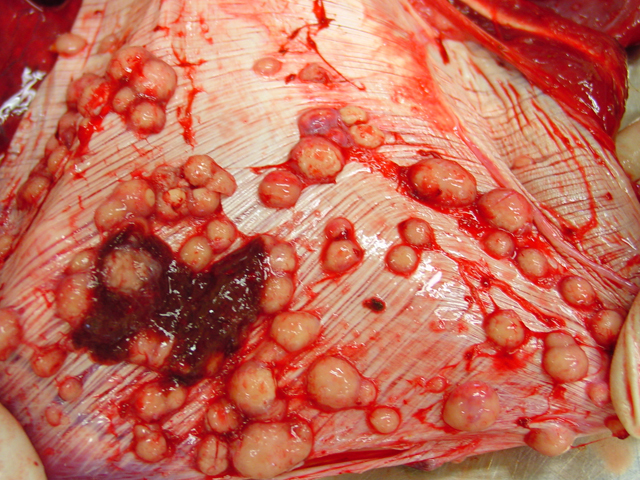
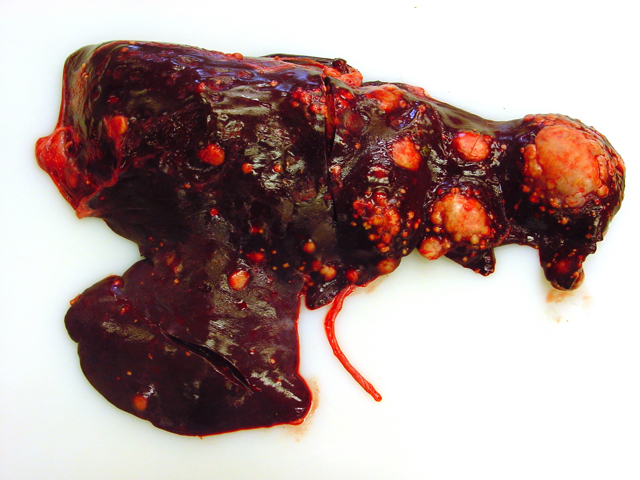
Firm, whitish nodules in size from
1 to 7 centimeters in diameter were found in the liver,
lung, mediastinum, pleura, and omentum (
Figs. 4-1,
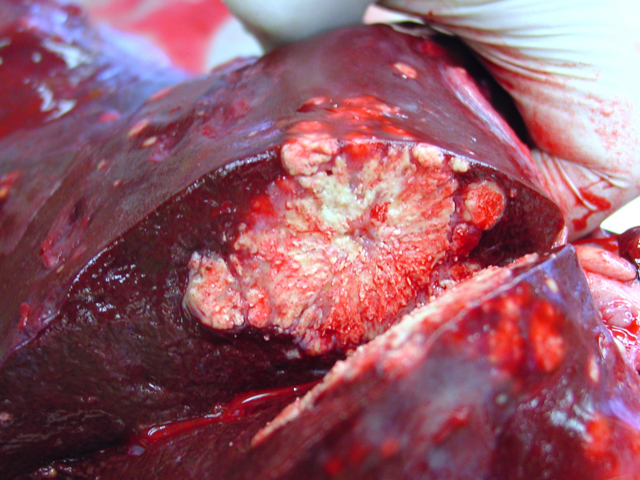
4-2). In the cut surface some of the nodules showed fine
whitish to grey beige spikes which were arranged in radial
patterns (
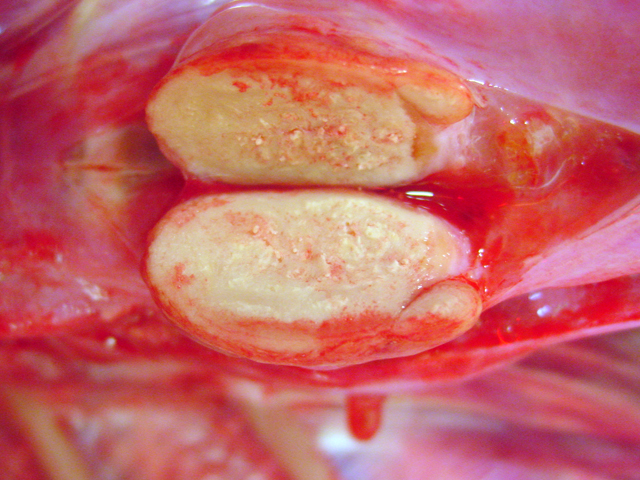
Fig. 4-3), other nodules showed centrally located
homogenous grey beige caseous material with multifocal
white gritty spots (
Fig. 4-4).
Histopathologic Description:
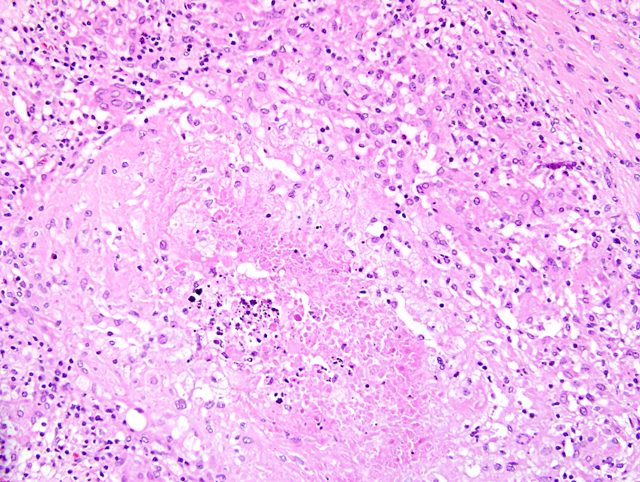
Liver: Up to
70% of the parenchyma is replaced by multifocal to
coalescing granulomas characterized by an amorphous,
eosinophilic necrosis material surrounded by a smaller
layer of elongated macrophages with light eosinophilic
cytoplasm, a centrally located oval nucleus and indistinct
cell boarders (epithelioid cells) and rarely of large cells
with eosinophilic cytoplasm and numerous nuclei located
in the periphery of the cell (multinucleated giant cells,
Langhans-type) which are once again surrounded by a rim
of lymphocytes, plasma cells, macrophages, fibroblasts
embedded in a small amount of collagen fibers and to a
lesser extend neutrophils (
Fig. 4-5). Multifocally necrotic
areas contain dark basophilic granular material (dystrophic
calcification). The liver parenchyma is diffusely infiltrated
with a moderate amount of lymphocytes and neutrophils.
Morphologic Diagnosis:
Liver,
granulomatous hepatitis, multifocal and coalescing,
severe, chronic, with central caseous necrosis and myriads
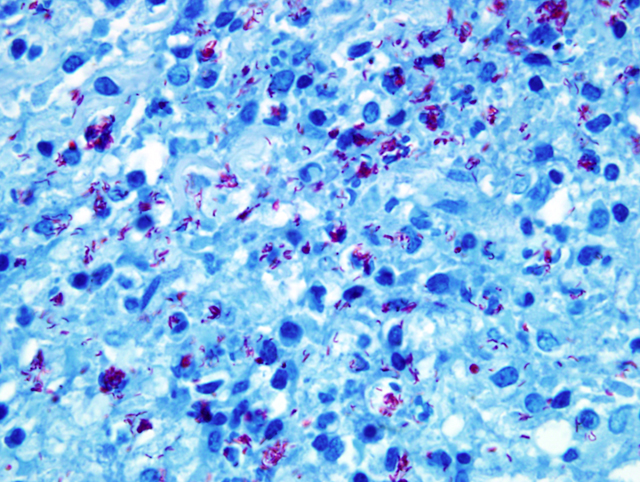
of acid-fast intrahistiocytic rod shaped bacteria (
Fig. 4-6)
(
Mycobacteria kansasii)
Lab Results:
Acid-fast stain (Ziehl-
Neelson stain) revealed myriads of acid-fast bacteria in
epithelioid macrophages and the PCR analysis resulted in
Mycobacterium kansasii.
Condition:
Mycobacterium kansasii
Contributor Comment:
Mycobacterium kansasii
is classified in Runions Group 1 and belongs to the
nontuberculous mycobacteria (NTM); or mycobacteria
other than tuberculosis (MOTT)). It is a saprophyte which
is found in soil and water.Â
M. kansasii is very heterogeneous
as 5 well-defined different types (I-V) exist of which type
I is frequently isolated from humans, type II from humans
and the environment and III-V were present mostly in
the environment and only rarely in humans sources.
2 M.
kansasii infection is described in cattle to cause lesions
similar to bovine tuberculosis (BTB) although it seems
to be exceedingly rare.
5,6 In slaughtered cattle in Great
Britain an incidence of 0.8% NTM was found and the
majority of these belong to the M. avium complex.
5 In
Northern Ireland
M. kansasii could be detected in 14
tissue specimens of 16,506 cattle, which demonstrates
the rareness of the disease in cattle. Nevertheless, it is not
known how many animals that are exposed to
M. kansasii
do not develop disease. It was also found that
M. kansasii
could be isolated from humans without disease.
5 However,
M. kansasii can cause pulmonary or disseminated disease
in humans with an estimated 300 times higher incidence
in HIV- patients.
2 Experimental infection of healthy cattle
failed to cause disease or pathologic changes but it did
induce immune responses in TB tests.
6 Since, some NTM
and specifically
M. kansasii do share some diagnostic
antigens with
Mycobacterium bovis complex bacteria
4, 5
there can be cross-reactions in traditional TB tests, which
complicates the control and eradication of BTB.
JPC Diagnosis:
Liver: Granulomas, multifocal to coalescing, with mild hepatocellular degeneration
Conference Comment:
Mycobacteria are grampositive
bacteria with high lipid content within their
cell wall. This makes traditional gram staining largely
ineffective, but mycobacteria do stain with carbol-fuschin
and resist decoloration by inorganic acids giving them
their acid-fast staining characteristic.
3 Mycolic acid
spacing within the bacterial cell wall is key to these
bacteria being acid-fast.
3 These same mycolic acids are
hydrophobic accounting for the environmental hardiness
and antimicrobial resistance of these troublesome bacteria.
3
During the conference, Colonel Raymond questioned the
participants about the classifications of mycobacteria
and then the mechanistic basis of tuberculin skin testing.
These bacteria cause a type IV hypersensitivity reaction,
or delayed type hypersensitivity, as demonstrated by the
tuberculin test. Histologically this manifests as aggregates
of mononuclear cells around small veins and venules,
or perivascular inflammation and cuffing.
1 Important
cytokines involved in delayed type hypersensitivity
reactions include IL-12, IL-2, IFN-gamma, and TNF. IL-
12 is critical in propagating a Th1 response, IL-2 causes
an autocrine and paracrine proliferation of T cells, and
IFN-gamma is a potent macrophage activator.1 TNF is an
important cytokine that acts on endothelial cells to cause
vasodilation and facilitates the process of adhesion and
extravasation of lymphocytes and monocytes.
1
References:
1. Abbas, Abul K: Disease of Immunity.Â
In: Robins
and Cotran Pathologic Basis of Diseases, ed. Kumar
VK, Abbas AK, Fausto N, 7th ed., pp. 216-218. Elsevier,
Philadelphia, Pennsylvania, 2005
2. Alcaide F, Richter I, Bernasconi C, Springer B,
Hagenau C, Schulze-R+�-�bbecke R, Tortoli E, Martin
R, B+�-�ttger EC, Telenti A: Heterogeneity and clonality
among isolates of
Mycobacterium kansasii: implications
for epidemiological and pathogenicity studies. J Clin
Microbiol
35(8):1959-64, 1997
3. Caswell JL, Williams KJ: Respiratory system.Â
In: Jubb,
Kennedy and Palmers Pathology of Domestic Animals,
ed. Maxie MG, 5th ed., pp. 632. Elsevier, Philadelphia,
Pennsylvania, 2007
4. Huges MS, Ball NW, McCarroll J, Erskine M, Taylor
MJ, Pollock JM, Skuce RA, Neill SD: Molecular analysis
of mycobacteria other than the
M. tuberculosis complex
isolated from Northern Ireland cattle. Vet microbiol
108:101-112, 2005
5. Vordermeier HM, Brown J, Cockle OJ, Franken WPJ,
Arend SM, Ottenhoff THM, Jahans K, Hewinson RG:
Assessment of cross-reactivity between
Mycobacterium
bovis and
M. kansasii ESAT-6 and CFP-10 at the T-cell
epitope level. Clin Vaccine Immunol
14(9):1203-9, 2007
6. Waters WR, Palmer MV, Thacker TC, Payeur JB,
Harris NB, Minion FC, Greenwald R, Esfandiari J,
Andersen P, McNair J, Pollock JM, Lyashchenko KP:
Immune responses do defined antigens of
Mycobacterium
bovis in cattle experimentally infected with
Mycobacterium
kansasii. Clin Vaccine Immunol
13:611-9, 2006