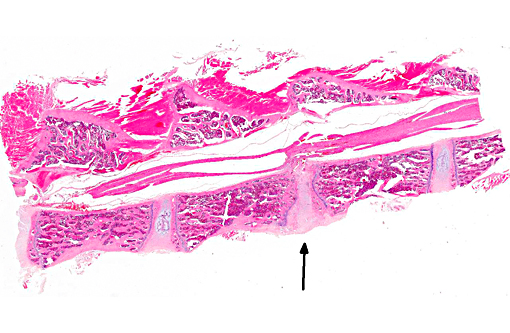
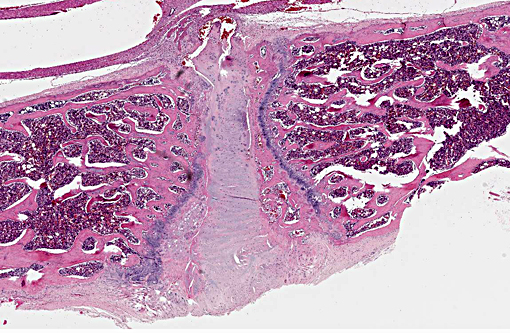
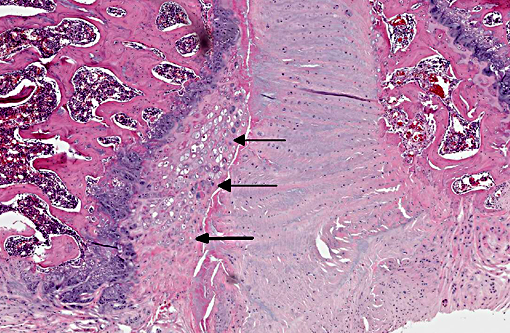
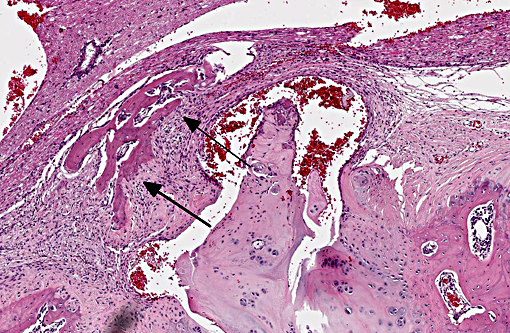
Signalment:
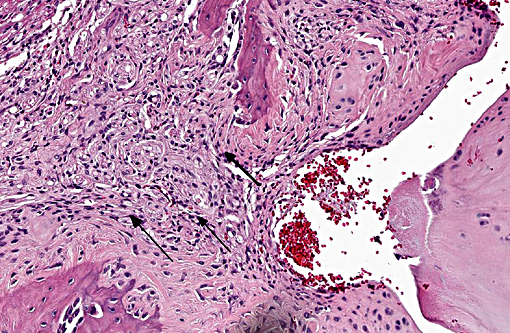
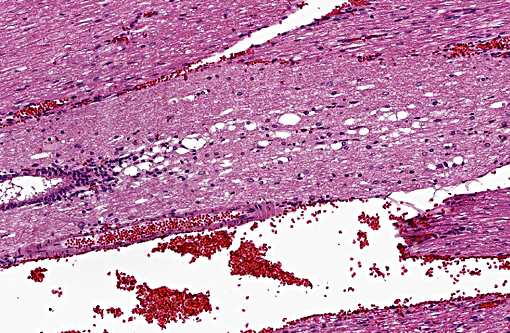
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
The normal intervertebral disc is avascular and primarily aneural. It is comprised of a dynamic extracellular matrix as well as a fibrocartilaginous network that maintains tensile strength. Importantly, there is normally a clear morphological distinction between the peripheral annulus fibrosus (AF) and inner nucleus pulposus (NP).9 The composition of the outer AF is primarily type I collagen fibers that are aligned at approximately 30° angles to the longitudinal axis of the spine. The direction of the type I fibers alternates in each concentric layer of the disc in an orientation designed to provide maximal strength. These collagen fibers also align with the cells of the AF, which are fibroblast-like with elongated nuclei. The inner layer of the disc, the nucleus pulposus (NP), contains a viscous proteoglycan gel, a small amount of type II collagen, and (in humans; see more below) chondrocyte-like, rounded cells.(5) The proteoglycan gel is primarily composed of aggrecan, which is extremely hydrophilic. The aggrecan absorbs water and creates a swelling pressure responsible for maintaining tissue hydration and balancing the osmolarity of the disc.(9) Consequently, a hydrostatic pressure is created within the nucleus, which is contained by the lamellae of the annulus, and distributes loads evenly across the underlying/adjacent vertebrae.(3)
Vertebral disc injury causes a degradative cascade that leads to both physiological and morphological changes. The degenerative changes involve increased breakdown of matrix, altered matrix synthesis, and cell loss through apoptosis. Fissures in the outer annulus may cause bulging of the disc(4) and aggrecan degeneration of the nucleus pulposus leads to infiltration of nerve endings and blood vessels into the normally avascular disc, resulting in pain. Loss of aggrecan also causes a decrease in hydration and swelling pressure, causing decreases in disc height and alterations in load carrying capacity.(9)
In a damaged disc, there eventually is no clear morphologic distinction between the AF and the NP. Over time, the organization of the chondrocyte-like NP cells is lost as these cells are replaced by fibrosis characterized by decreased type II collagen and increased type I and type X collagen.(4,5) The predictable pattern of the AF cells becomes disorganized as cells are lost or become arranged in clusters.(9) Finally, disc injury also causes the recruitment of inflammatory cytokines and degradative enzymes. IL-1, the primary cytokine in the damaged disc, inhibits the production of the extracellular matrix and stimulates additional cytokine production.(5) As additional cytokines are recruited to the damaged disc, apoptotic cell death ensues. There are several differences in the development, cell composition, anatomy, physiology, and mechanical properties of intervertebral discs between humans and animals.(1) The composition of the nucleus pulposus and the organization of the cartilage endplates are two notable examples of differences between humans and many animal species. Throughout the life of rats the nucleus pulposus is composed of notochordal cells, which are large, highly vacuolated, loosely arranged, produce large quantities of hyaluronin, and influence the metabolism of other cell types within the disc. In contrast, numbers of notochordal cells decrease rapidly in the nucleus pulposus of young humans and in adults are replaced by chondrocytic cells.
In rats and many other species the secondary ossification centers of the vertebral body form a complete osseous epiphyseal plate between the intervertebral disc and the vertebral body physis and this persists throughout the life of the animal. In humans, however, the epiphysis from the secondary ossification center exists as a ring adjacent to the outer annulus fibrosus rather than as a complete plate adjacent to the disc and the physis closes at about 25 years of age. Because of this the base of the cartilage endplate acts as the growth region for the vertebral body in humans while in most other species vertebral body growth occurs via the epiphyseal plates. The relevance and translatability to humans of results obtained from studies of disc degeneration using animal models must consider the potential impact of differences such as these.
JPC Diagnosis:
Conference Comment:
Degeneration of intervertebral discs is a particularly important disease not only in people, but also in dogs. It occurs in all dog breeds but there are significant differences in the pathogenesis between chondrodystrophic and nonchondrodystrophic breeds. Chondro-dystrophic breeds have an increased expression of fibroblast growth factor 4 (FGF4), which affects not only appendicular skeleton formation, but also the composition of the nucleus pulposus (NP) in their intervertebral discs. In these breeds the NP contains a much greater content of collagen than proteoglycan, and collagen content can increase over time in the degeneration process, decreasing water content and the discs ability to adequately absorb mechanical forces. The NP begins to degenerate very early in life, and microscopically this is seen as chondrocyte proliferation and lobulation of the NP. These changes occur in all intervertebral discs. Eventually the NP with higher collagen content begins to degenerate, harden and may mineralize, becoming friable, and the inner aspects of the annulus fibrosis (AF) can also begin to degenerate and tear, allowing fragments of NP to enter the AF. These changes can extend through the AF, resulting in a Hansen type I intervertebral disc herniation, with fragments of degenerate NP extending through the dorsal longitudinal ligament into the spinal canal. This most frequently results in local myeomalacia in the affected spinal cord region with accompanying inflammation, but can result in ascending myelomalacia in some severe cases.(2)
Nonchondrodystrophic breeds do not suffer the same type of degeneration and mineralization of the NP as chondro-dystrophic breeds. The NP in their intervertebral discs may undergo degeneration and fibrous metaplasia, but it occurs later in life and generally only affects a single disc or few discs are involved, and the degeneration is thought to be related to trauma and disruption of the annulus fibrosis. These changes result in a Hansen type II herniation which refers to partial hernitation of the NP through the AF, bulging of the AF, and compression of the dorsal longitudinal ligament, which may then impinge on the spinal cord. Type II herniations in general result in less severe disease. Disc herniations most commonly occur dorsally or dorso-laterally, but can also occur ventrally, cranially and caudally. Cranial and caudal herniation of the nucleus pulposus into the vertebral body creates a lesion known as a Schmorls node within the vertebral endplate. Intervertebal disc herniations are rare in other species but have been documented in the cervical region of horses, similar to type II lesions in dogs. Sows and boars have also been documented to have degenerative intervertebral disc changes, but not dorsal herniation of the NP. (2)
References:
1. Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008; 17:2-19.
2. Craig LE, Dittmer KE, Thompson KG. Bones and Joints. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol1. St. Louis, MO: Elsevier; 2015:142-147.
3. Ferguson SJ, Steffen T. Biomechanics of the aging spine. Eur Spine J. 2003; 12:S97.103.
4. Keorochana G, Johnson J, Taghavi C, et. al. The effect of needle size inducing generation in the rat caudal disc: evaluation using radiograph, magnetic resonance imaging, histology, and immunohistochemistry. The Spine Journal. 2010; 10: 1014-1023.
5. Kepler C, Ponnappan R, Tannoury C, et. al. The molecular basis of intervertebral disc degeneration. The Spine Journal. 2013: 318-330.
6. Phillips FM, Reuben J, Wetzel FT. Intervertebral disc degeneration adjacent to a lumbar fusion: an experimental rabbit model. J Bone Joint Surg Br. 2002; 84: 239-294.
7. Singh K, Masuda K, An H. Animal models for human disc degeneration. The Spine Journal. 2005; (5): 267S-279S.
8. Sun F, Qu J, Zhang Y. Animal models of disc degeneration and major genetic strategies. Pain Physician. 2013; (16): E267-E275.
9. Urban J, Roberts S. Degeneration of the intervertebral disc. Arthritis Research and Therapy. 2003; (5): 120-130.