Signalment:
Gross Description:
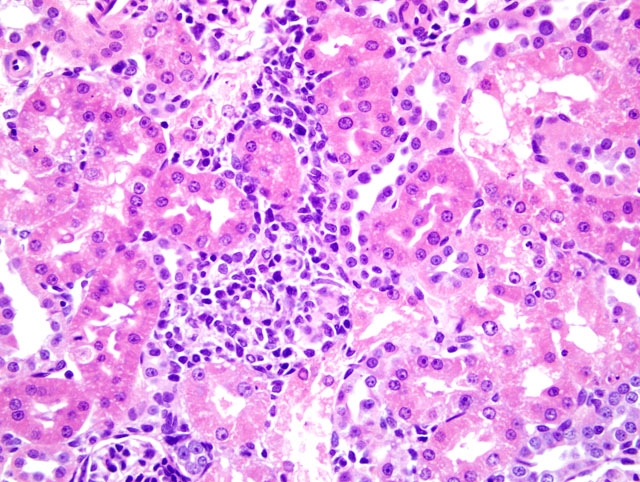
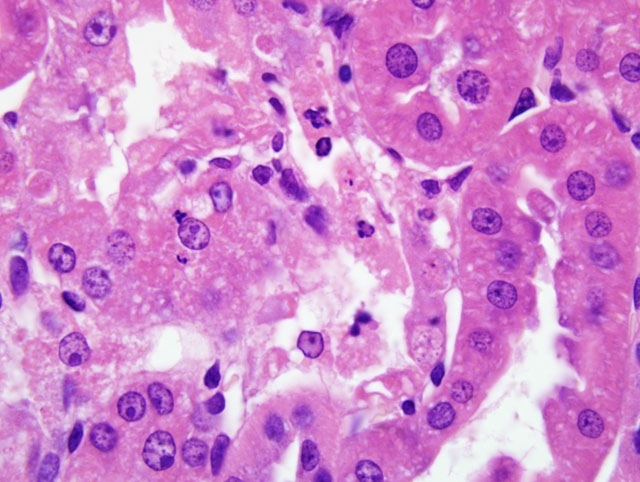
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
The key histopathological features of GM-treated animals are vacuolar degeneration and necrosis of renal proximal convoluted tubular epithelium in the cortex.(4,6) Ultrastructual changes of proximal tubule epithelial cells include damage and loss of brush borders, and formation of cytosegrosomes and myeloid bodies. (4,6) Gentamicin nephrotoxicity is reversible and regeneration of tubular epithelium may occur even during continued therapy. (4,6)
The underlying mechanism by which GM causes nephrotoxicity is not fully understood. However, one of the most important mechanisms for GM induced nephrotoxicity is reactive oxygen species-mediated. (2,5) It has been shown that GM generates reactive oxygen species which produce cellular injury and necrosis via peroxidation of membrane lipids, protein denaturation, and DNA damage in the kidney.(1,2) It has also been reported that GM suppresses antioxidant defense enzyme (e.g. superoxide dismutase, catalase and glutathione peroxidase) and increases lipid peroxidation in the renal cortex and medulla.(2,5)
A recent interesting development is the attempt to use extracts from medicinal plants with antioxidant properties to ameliorate/protect against GM-induced nephrotoxicity in rats. Such medicinal plants include garlic, as well as diallyl sulfide, a compound isolated from garlic, Nigella sativa oil, Maidenhair tree extract (Ginkgo biloba), Rhazya stricta and green tea extract.(1,5,8)
JPC Diagnosis:
Conference Comment:
A focal point of discussion for conference participants was the histology of the rat kidney in relation to lesion distribution. Histologically, the rat kidney can be divided into five zones. Beginning with the capsular surface, these are: the cortex; the outer stripe of the outer medulla; the inner stripe of the outer medulla; the inner medulla; and the papilla. The proximal tubule is often subdivided into three segments: P1 and P2 contain the proximal convoluted tubule (PCT); and P3 represents the pars recta. While P1 and P2 are located in the cortex, the P3 segment is found in the outer stripe of the outer medulla. Accurate classification of renal lesions with respect to the structures affected and anatomic location can aid in determination of potential causes of renal tubular damage and associated mechanisms of action. The proximal tubule is often the site of toxic injury, with P3 most commonly affected. The histologic lesion patterns of acute renal tubular necrosis include: multifocal distribution, affecting individual cells or groups of tubules, such as with N-(4-fluoro-4-biphenyl) acetamide; segmental distribution, such as when affecting only P3 (aminoglycosides, furans, thiophenes, acetaminophen, ochratoxin A and mercuric chloride); and multiple proximal tubule segments, such as that which occurs with uranyl nitrate and cisplatin.(7)
The moderator commented on the sexual dimorphism exhibited in rat kidneys. Cells within segments P1 and P2 in the male rat exhibit higher endocytic activity and have larger, more numerous lysosomes. Within the PCT of sexually mature males, cells frequently have eosinophilic cytoplasmic granules composed of alpha2U-globulins; visualization of these granules is enhanced by staining with Mallory-Heidenhain stain.(3) Abnormal accumulation of these granules is referred to as hyaline droplet nephropathy and can be seen in male rats as a result of toxic changes, or in both sexes in association with disseminated histiocytic sarcoma.(3) Female rats have more extensive smooth endoplasmic reticulum in segments P1 and P2, which suggests higher mixed-function oxidase activity and enhanced metabolism of drugs.(7)
References:
2. Banday AA, Farooq N, Priyamvada S, Yusufi, ANK, Khan F. Time dependant effects of gentamicin on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in rat kidney. Life Sci. 2008;82:450-459.
3. Hard GC, Alden CL, Bruner RH, Frith CH, Lewis RM, Owen RA, Krieg K, Durchfeld-Meyer B. Non-proliferative lesion of the kidney and lower urinary tract in rats. In: Guides for Toxicologic Pathology; accessed at http://www.toxpath.org/nomen/index.htm, 11 August 2010. Washington D.C.: Society of Toxicologic Pathologists/American Registry of Pathology/The Armed Forces Institute of Pathology; 1999.
4. Houghton DC, Harnett M, Campbell-Boswell M, Porter G, Bennett WM. A light and electron microscopic analysis of gentamicin nephrotoxicity in rats. Am J Pathol. 1976;82:589612.
5. Khan SA, Priyamvada S, Farooq N, Khan S, Khan MW, Yusufi ANK. Protective effect of green tea extract on gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Pharmacol Res. 2009;59:254-262.
6. Mingeot-Leclerq M, Tulkens PM. Aminoglycosides: nephrotoxicity. Antimicrob Agents Chemother. 1999;43(5):10031012.
7. Montgomery CA Jr., Seely JC: Kidney. In: Boorman GA, Eustis SL, Elwell MR, Montgomery CA, MacKenzie WF, eds. Pathology of the Fischer Rat. San Diego, CA: San Diego Academic Press; 1990:127-153.
8. J. Pedraza-Chaverri, P.D, Maldonado, O. Medina-Campos, et al. Garlic ameliorates gentamicin nephrotoxicity: relation to antioxidant enzymes. Free Radic Biol Med. 2000;29(7):602-611.