Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 20
April 6th, 2018
CASE I: T16-13553 (JPC 4084197).
Signalment: 10-year-old, female, spayed, American bulldog (Canis familiaris), canine.
History: Acute (sudden death). No additional history was available. A formalin-fixed tissue described as from a gallbladder mass was received.
Gross Pathology: Abdominal (gallbladder mass) was observed on gross examination.
Laboratory Results (clinical pathology, microbiology, PCR, ELISA, etc.): None provided.
Microscopic Description:
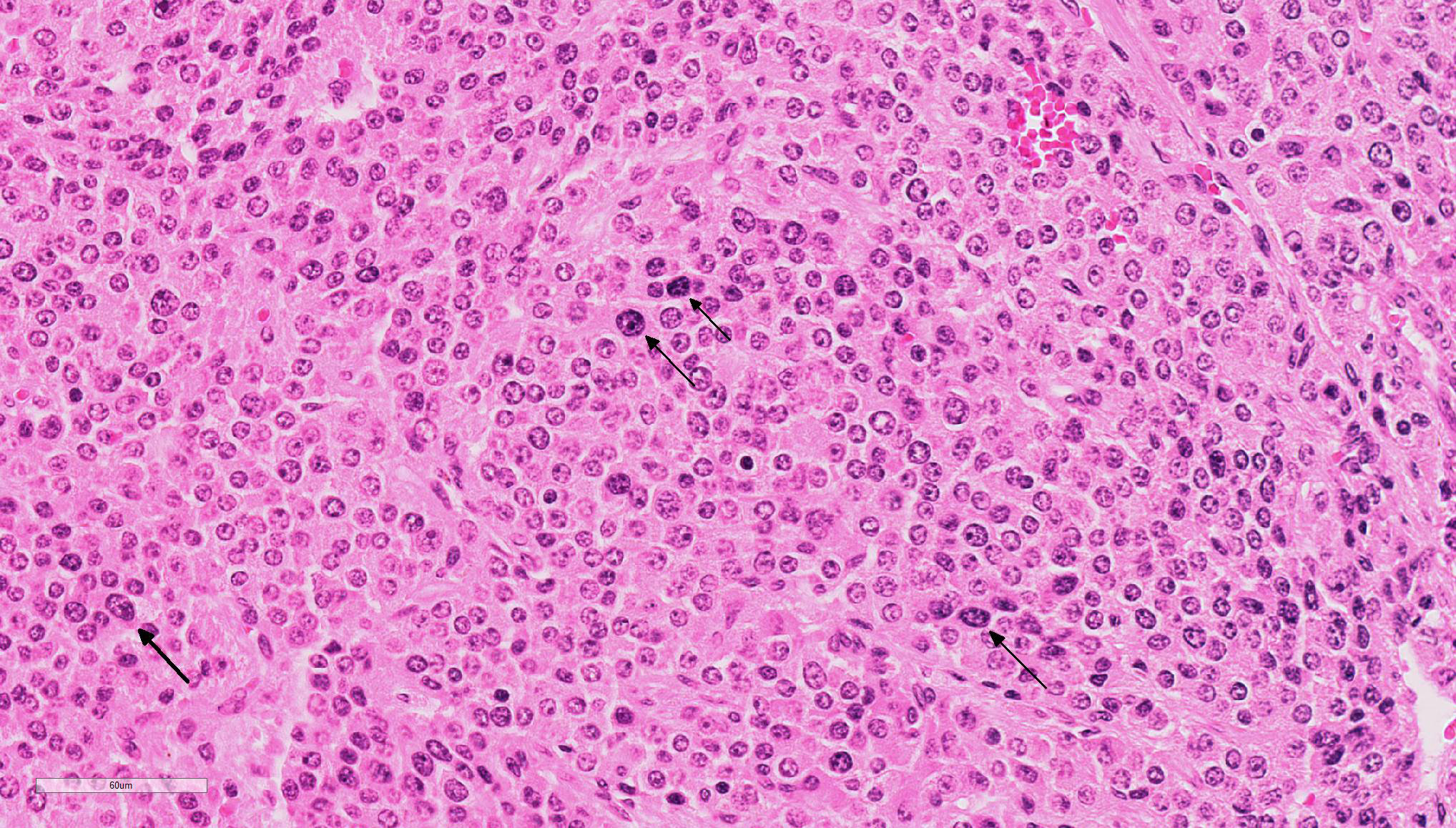
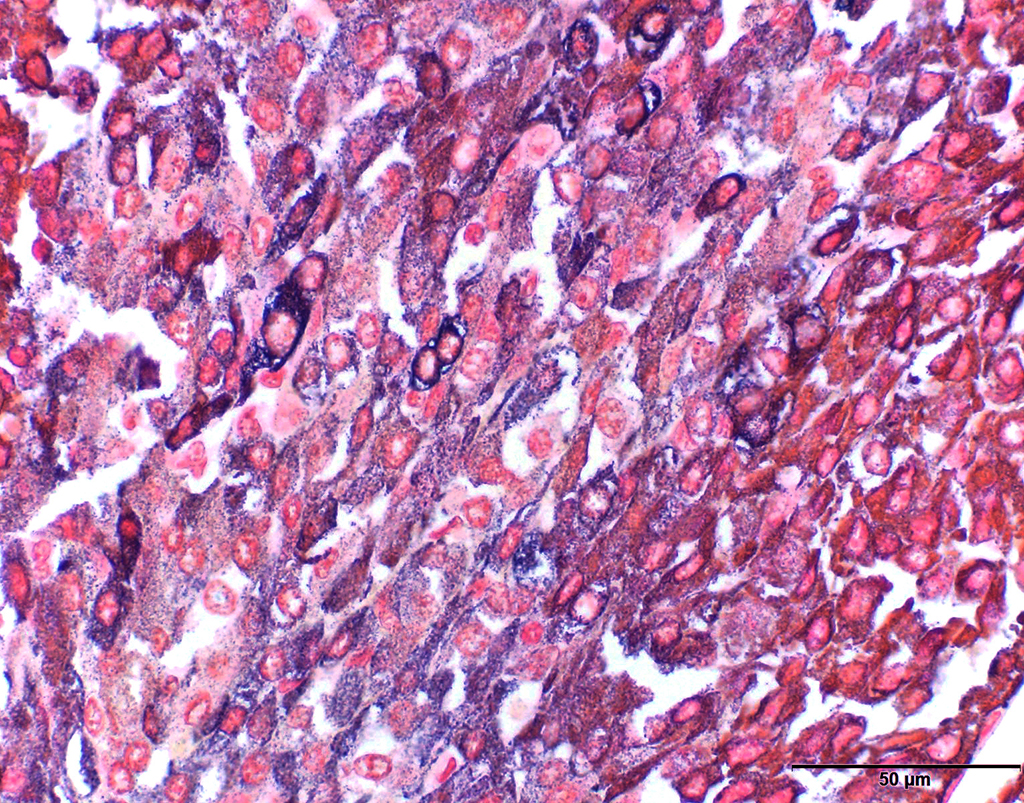
A formalin-fixed tissue from a gallbladder mass was received. A non-demarcated, non-capsulated neoplasm effaced the architecture of the gall bladder. The neoplasm is composed of closely packed sheets and nests of round cells supported on fine to ample fibrovascular stroma The neoplastic cells had indistinct cell borders and moderate, occasionally vacuolated, finely granular, eosinophilic cytoplasm. The nuclei were round, variably sized and had stippled nuclear chromatin. Occasional nuclei were hyperchromatic. Nucleoli were small to indistinct. Mitotic cells were very rare (<1 per 10x HPF). Anisocytosis and anisokaryosis were mild to moderate. Multifocally, aggregates of lymphocytes were observed in some sections. Dark staining cytoplasmic granules were demonstrated by special stains (Churukian-Schenk) for argyrophilic granules.
Contributor’s Morphologic Diagnosis:
Neoplasm, neuroendocrine tumor (carcinoid), gallbladder
Contributor’s Comment: Carcinoid tumors are uncommon neoplasms in humans and animals. The tumors arise from dispersed cells of the neuroendocrine system in the gastrointestinal tract, biliary system, pancreas and lungs. In dogs, hepatic, gastrointestinal and pulmonary carcinoids have been reported. Carcinoid tumors are reported in both sexes, several different breeds, and over an age range of 9-13 years. Extrahepatic biliary (gallbladder) carcinoid tumors are rare tumors in humans and very rare in dogs.3,5,6,8 Carcinoid tumors in humans are potentially malignant and are usually slow growing tumors with a 5-year survival rate of 94% if localized, 64% if regional metastasis is present and 18% if distant metastasis has occurred during diagnosis.7 Metastasis of carcinoids is also common in domestic animals and demonstrating the malignancy of the tumor. The tumor metastasizes in a similar manner to adenocarcinomas, through lymphatics and hematogenous routes. In dogs, small intestinal carcinoids frequently spread to the lung and pleura, liver, regional lymph nodes and pancreas.3
Since reports on canine primary gallbladder carcinoid are very rare, adequate information is unavailable to determine malignancies of primary gallbladder carcinoids in dogs. However, in one report, the presence of neoplastic cells within vessels of the gallbladder was suggested to indicate the potential of canine gallbladder carcinoid for metastatic dissemination.8 Carcinoid tumors release various secretory products that often govern a clinical syndrome. For example, vasoactive amines released from the tumors may result in diarrhea, skin flushing, or cyanosis, hypertension, bronchoconstriction, pulmonary valvular stenosis, and right heart failure. Typical carcinoid syndrome as reported in humans has not been reported in domestic animals.3
Carcinoid tumors are commonly misdiagnosed especially if the tumor occurred in uncommon sites. Definitive diagnosis of carcinoid tumors requires immunohistochemistry, or special stains for argyrophilic granules or electron microscopy.8 Immunohistochemistry against chromogranin A, synaptophysin and neuron specific enolase are often applied. The cytoplasmic granules stain positive with argyrophilic stains such as Churukian-Schenk and Grimelius stains. Therefore, special stains, such as Churukian-Schenk and Grimelius stains, which are readily available, are relatively inexpensive and should be applied in suspected cases of a carcinoid tumor.
JPC Diagnosis: Gallbladder: Carcinoid, American bulldog (Canis familiaris), canine.
Conference Comment: Carcinoids arise from neuroendocrine cells within mucosa of various organs, but most often the stomach and intestine. In animals, those neuroendocrine cells either secrete low-molecular-weight polypeptide hormones like secretin, somatostatin, and cholecystokinin or are part of a larger amine precursor uptake decarboxylation (APUD) group which produces serotonin among other compounds. Although carcinoids are rare in animals, the most commonly reported locations in dogs are the duodenum, colon, and rectum. They are even more scarcely reported in other organs such as the lungs (more common in humans), liver, and gallbladder as in the present case.2,11 As noted above by the contributor, there have been three recent reports of gallbladder carcinoids, all in dogs.5,8 Historically, they have also been reported in the gallbladder of cats and cattle.
Within the liver, carcinoids arise from the neuroendocrine cell population associated with biliary epithelium and hepatic parenchyma; therefore tumors may form within the liver, extrahepatic bile ducts, or gallbladder.2 Clinically, carcinoids most frequently cause secondary bowel obstruction and anemia from ulceration with subsequent hemorrhage. They can also be easily mistaken for polyps, especially when located at the anorectal junction. Diarrhea may be another associated finding, as a result of release of functional polypeptide hormones.11
Grossly, carcinoids are lobulated, firm, dark red to tan, and rarely larger than a few centimeters in diameter. They arise deep within the mucosa, commensurate with the location of neuroendocrine cells, causing submucosal nodules with ulceration and erosion of the overlying mucosa and often infiltrating transmurally into the mesentery.11 In the liver, there is frequent intrahepatic spread with metastasis to local lymph nodes, peritoneum, and lung.2
Microscopically, carcinoids are composed of round to oval polygonal cells with abundant amounts of finely granular eosinophilic to finely vacuolated cytoplasm with round vesiculate nuclei and prominent nucleoli. In typical neuroendocrine fashion, the cells are arranged in nests, and packets expanding the mucosa, submucosa, and muscularis; packets are surrounded by a fine fibrovascular stroma. Additional findings include: amyloid in between cells and adjacent to stromal blood vessels and megalocytes or multinucleated neoplastic cells. A potential histologic differential diagnosis is intestinal mast cell tumor, owing to the presence of numerous granules.
Ultrastructurally, carcinoid cells have numerous dense, round to oval, variably sized, membrane-bound intracytoplasmic secretory granules with abundant rough endoplasmic reticulum (RER) and a plasma membrane with interdigitating processes.11 Mast cell tumors have less dense granules that can vary from homogenous to scroll-like admixed with large clear vacuoles (degranulation) and less RER.
The diagnosis of carcinoids is based on the following: neuroendocrine microscopic pattern, cytoplasmic granules which are argentaffinic (reduce silver solution to metallic silver after formalin fixation) and argyrophilic (reduce silver solution to metallic silver after being exposed to a pre-reduction step)10 granularity, immunohistochemical identification of secretory products, and unique ultrastructural appearance. Histochemical and immunohistochemical (IHC) reactivity can vary, especially if there is significant autolysis prior to fixation (common in the gastrointestinal tract). However, in decent quality specimens, carcinoid cells are positive for neuron-specific enolase (NSE) and chromogranin as well as immunopositive for peptides being produced (e.g. serotonin). Those peptides may also be identified in circulation further supporting an endocrine diagnosis. Carcinoid cells are negative for periodic acid-Schiff and the granules do not exhibit metachromasia with Giemsa stains, further differentiating carcinoid from intestinal mast cell tumor.11
In humans, several paraneoplastic syndromes have been identified, such as: cutaneous flushing, diarrhea, bronchospasm, and systemic fibrosis. Fibrosis is concerning as it can lead to dysfunction of the pulmonary and tricuspid valves followed by regurgitation and right heart failure. This syndrome, termed “carcinoid syndrome”, is most common in patients that develop carcinoids in their respiratory tract. The right side of the heart is affected because monoamine oxidases in the lungs fail to inactivate vasoactive substances, and these vasoactive substances lead to the aforementioned cutaneous flushing and bronchospasm which are key clinical indicators.9 The mechanism of fibrosis in humans is unclear but is postulated to result from interactions between tumor cells and fibroblasts or hormones and peptides secreted by the carcinoid.4
Conference participants discussed the differences between benign and malignant carcinoids, which, according to the moderator, are much more common in the liver (in his experience). In this case, with a history of a single mass and no evidence of vascular invasion in the submitted sections, this carcinoid is likely benign. Additionally, attendees reviewed the origin of carcinoid tumors and the moderator cited an article1 which stated that proliferating cholangiocytes may take on a neuroendocrine phenotype, as well as initiate vessel proliferation, which may facilitate metastasis.1 Shunt dogs, for example, tend to have proliferation of cholangiocytes and arterioles at the same time.
Contributing Institution:
The University of Georgia
College of Veterinary Medicine
Department of Pathology
Tifton Veterinary Diagnostic and Investigational Laboratory
Tifton, GA 31793, USA
http://www.vet.uga.edu/dlab/tifton/index.php
References:
- Alvaro D, Mancino MG, Glaser S, Gaudio E, et al. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. 2007; 132(1):415-431.
- Cullen JM, Stalker MJ. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 2. 6th ed. St. Louis, MO: Elsevier; 2016:349.
- Head KW, Else RW, Dubielzig RR. Tumors of the alimentary tract. In: Meuten DJ, ed. Tumors in Domestic Animals. 4th ed. Ames, IA: Iowa State Press; 2002: 468-470.
- Laskaratos FM, Rombouts K, Caplin M, Toumpanakis C, Thirlwell C, Mandair D. Neuroendocrine tumors and fibrosis: An unsolved mystery?. 2017; 123(24):4770-4790.
- Lippo NJ, Williams JE, Brawer RS, Sobel KE. Acute hemobilia and hemocholecyst in 2 dogs with gallbladder carcinoid. J Vet Intern Med. 2008; 22 (5):1249-52.
- Mezi S, Petrozza V, Schillaci O, et al. Neuroendocrine tumors of the gallbladder: a case report and review of the literature. J Med Case Reports. 2011; 5:334
- Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997; 79:813-829.
- Morrell CN, Volk MV, Mankowski JL. A carcinoid tumor in the gallbladder of a dog. Vet Pathol. 2002; 39 (6):756-8.
- Shinn BJ, Tafe LJ, Vanichakarn P. A case of carcinoid syndrome due to malignant metastatic carcinoid tumor with carcinoid heart disease involving four cardiac valves. Am J Case Rep. 2018; 19:284-288.
- The internet pathology laboratory for medical education. Hosted by The University of Utah Eccles Health Science Library. https://library.med.utah.edu/WebPath/HISTHTML/STAINS/STAINS.html . Published 1994. Updated 2018. Accessed March 29, 2018.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 2. 6th ed. St. Louis, MO: Elsevier; 2016:105-106.