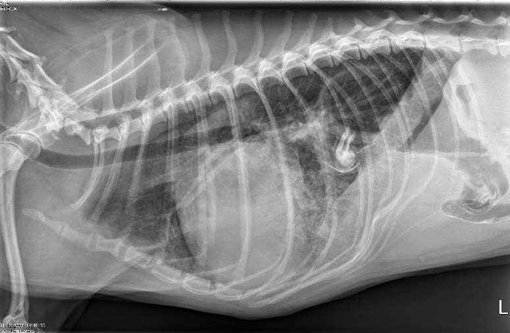
Signalment:
Gross Description:
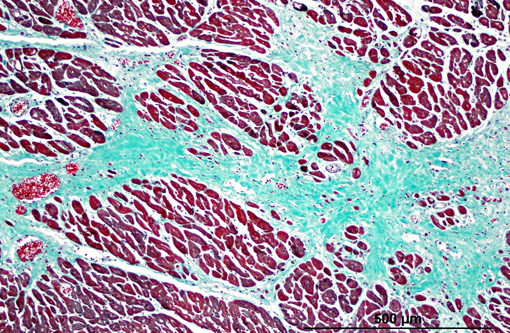
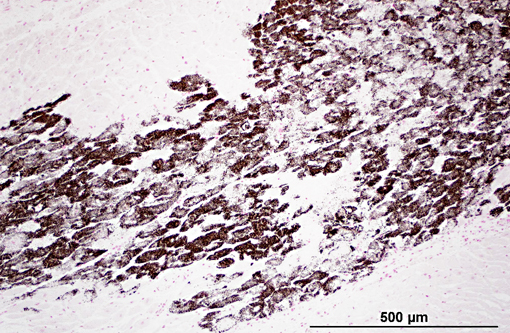
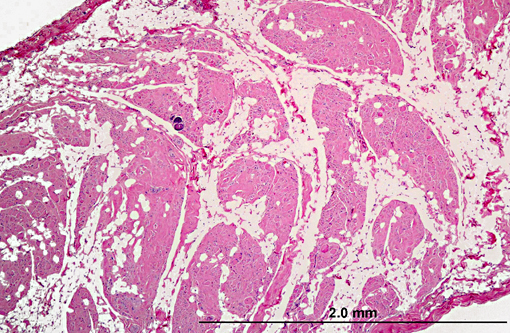
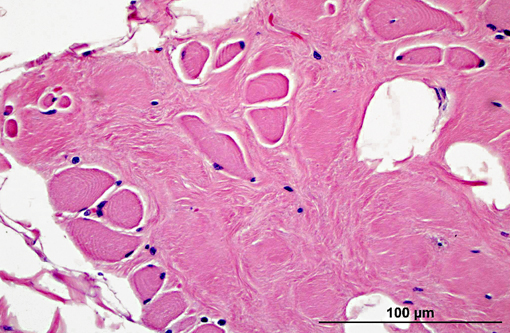
Histopathologic Description:
Diaphragm: The diaphragm is markedly thickened. Myofiber numbers are diffusely and markedly reduced while remaining myofibers are individualized by marked fibrosis and adipose tissue deposition. Myofibers vary markedly in diameter and frequently exhibit loss of cross striations, hyalinization, and fragmentation (degeneration). Scattered myofibers contain abundant granular basophilic material (mineral) within the sarcoplasm.
Morphologic Diagnosis:
1. Heart: myocardial mineralization and necrosis, multifocal to coalescing, moderate to marked, with moderate fibrosis
2. Diaphragm: myocyte necrosis and loss, multifocal to coalescing, marked with marked fibrosis and fat replacement.
Condition:
Contributor Comment:
X-linked dystrophin deficiencies have been identified in cats and a variety of dog breeds, including Irish Terrier, Samoyed, Rottweiler, Dalmation, Shetland Sheepdog, Labrador Retriever, Brittany Spaniel, Rat Terrier, Belgian Groenendael Shepherd, Schnauzer, and Spitz, but are best studied in the mdx mouse, German Shorthair Pointer, and Golden Retriever(11). This particular patient was a member of the breeding colony at the National Center for Canine Models of Duchenne muscular dystrophy (NCDMD) at the University of North Carolina-Chapel Hill. As the murine models fail to show severe clinical signs or cardiac lesions comparable to those observed in humans, these canine models fill an important niche in therapeutic studies(5,12).
Muscular dystrophies have been identified in people resulting from deficiencies or alterations of more than 30 different proteins(10) and may be X-linked, autosomal recessive, or autosomal dominant8. Many of these deficiencies have not yet been observed in veterinary species outside of the laboratory. Examples of spontaneous muscular dystrophies that have been identified in animals are summarized in table 1. Other references contain a more extensive list of murine musculodystrophy models(2,10).
Table 1. Spontaneous muscular dystrophies in veterinary speciesmodified from 8
| Deficient Protein (Human Disease name) | Protein Location | Species |
| Laminin α2 (Congential muscular dystrophy type 1A) (RC) | Extracellular Matrix | Dog(8) Cat(4, 6, 8) dy/dy mouse(8, 9) |
| α-, β-, γ-, or δ-sarcoglycan (Limb-girdle muscular dystrophy types 2C-F) | Transmembrane | Dog(1, 10) Cat(7) BIO14.6 hamster(10) |
| Dysferlin (Limb-girdle muscular dystrophy type 2B) | Transmembrane | SJL-Dysf mouse(9) |
| Dystrophin (Duchenne muscular dystrophy, Becker muscular dystrophy) | Cytoskeleton | Dog Cat mdx mouse(8, 11) |
JPC Diagnosis:
1. Heart, myocardium: Degeneration, necrosis, and loss, multifocal, with myocardial fibrosis and mineralization.
2. Diaphragm: Myofiber atrophy and loss, diffuse, moderate with myocyte atrophy and marked fatty infiltration.
Conference Comment:
Gross lesions include severe degeneration indicated by pale streaks in the diaphragm, trapezius and sartorius muscles. In chronic cases, the diaphragm is thickened, contracted, and fibrotic. In the heart, the left ventricular wall and right side of the interventricular septum are most severely affected by necrosis, mineralization, and fibrosis. Common histologic findings not seen in this case are the presence of swollen and dark-staining fibers (large dark fibers), which represent the earliest stages of degeneration. Muscle fiber necrosis is due to the influx of calcium through defects in the sarcolemma. The basal lamina of necrotic fibers is preserved, leaving an empty sarcolemmal tube capable of fully regenerating the myofibers(13).
Other common examples of muscular dystrophy in veterinary species include X-linked muscular dystrophy in mixed-breed cats, which have similar clinical, gross, and histologic findings to those seen in dogs. Cats with muscular dystrophy are also prone to develop malignant hyperthermia associated with restraint or general anesthesia. Unlike the canine condition, cardiac involvement in cats is not common. Merino sheep have an autosomal recessive muscular dystrophy, and skeletal muscle of affected sheep expresses normal dystrophin. The major gross feature is the replacement of the intermedius, soleus, anconeus, and medial head of the triceps with mature adipose tissue. This disorder only affects type 1 muscle fibers, with initial lesions of type 1 fiber hypertrophy followed by myofibril loss and formation of sarcoplasmic masses at the center or periphery of the cell. Some fibers develop peripheral annular fibrils known as ringbinden. This condition is a result of loss of alpha-actinin and desmin proliferation, which forms the sarcoplasmic masses. It is important to distinguish true muscular dystrophies, which are inherited progressive myopathies, from other muscle disorders such as secondary nutritional degenerative myopathies(13).
There are four categories of reaction to muscle injury. Focal monophasic reactions result from an isolated single event; multifocal monophasic reactions result from a single injurious event that causes widespread lesions in the same phase of injury; focal polyphasic reactions are due to repeated mechanical injury at the same location; and multifocal polyphasic reactions are the result of continuous injury over a prolonged period of time such that lesions are widespread and exhibit various pathologic changes ranging from degeneration to necrosis to regeneration(13). This case is an example of a multifocal polyphasic (multiphasic) muscle lesion.
References:
2. Durbeej M, Campbell KP: Muscular dystrophies involving the dystrophin-glycoprotein comples: an overview of current mouse models. Curr Opin Genet Dev 12:349-61, 2002.
3. Emery AE: The muscular dystrophies. Lancet 359:687-695, 2002
4. Gaschecn F, Jaggy A, Jones B: Congenital diseases of feline muscle and neuromuscular junction. J Feline Med Surg 6:355-366, 2004.
5. Khurana TS, Davies KE: Pharmacological strategies for muscular dystrophy. Nature Reviews: Drug Discovery 2:379-390, 2003.
6. Poncelet L, Resibois A, Engvall E, Shelton GD: Laminin α2 deficiency-associated muscular dystrophy in a Maine coon cat. J Small Anim Pract 44:550-552, 2003.
7. Salvadori C, Vattemi G, Lombardo R, Marini M, Cantile C, Shelton GD: Muscular dystrophy with reduced β-sarcoglycan in a cat. J Comp Path 140:278-282, 2009
8. Shelton GD, Engvall E: Muscular dystrophies and other inherited myopathies. Vet Clin North Am Small Anim Pract 32:103-124, 2002.
9. Shelton GD, Engvall E: Canine and feline models of human inherited muscle disease. Neuromuscul Disord 15:127-138, 2005
10. Vainzof M, Ayub-Guerrieri D, Onofre PC, Martins PC, Lopes VF, Zilberztajn D, Maia LS, Sell K, Yamamoto LU: Animal models for genetic neuromuscular diseases. J Mol Neurosci 34:241-248, 2008
11. Valentine BA, McGavin MD: Skeletal muscle. In: Pathologic Basis of Veterinary Disease, eds. McGavin MD, Zachary JF, 4th ed., pp. 1026-1029, 1035-1037. Elsevier, St. Louis, MO, 2007
12. Valentine BA, Winand NJ, Pradhan D, Moise NS, deLahunta A, Korneygay JN, Cooper BJ: Canine X-linked muscular dystrophy as an animal model of Duchenne muscular dystrophy: a review. Am J Med Genet 42:352-356, 1992
13. Van Vleet JF, Valentine BA. Muscle and tendon. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. vol. 1, 5th ed. Philadelphia, PA: Elsevier; 2007:198-9, 210-16.