WSC 2023-2024 Conference 5, Case 2
Signalment:
4-year-old, male neutered Borzoi, canine (Canis lupus familiaris)
History:
The patient presented for unlocalized pain and frequent episodes of vocalization and was diagnosed with discospondylitis of the L3-L4 vertebrae. Surgical stabilization was performed and the dog was discharged with pain relief and empirical antibiotics. After initial improvement, the dog started to display pain and developed polyuria. Radiographs revealed a lytic lesion at the C4-C5 region. Azotemia with proteinuria developed, and cytological examination of the urine revealed numerous fungal hyphae. Culture of blood and urine was positive for Rasamsonia (Geosmithia) argillacea. Itraconazole therapy was implemented, but the patient declined rapidly and was euthanized.
Gross Pathology:
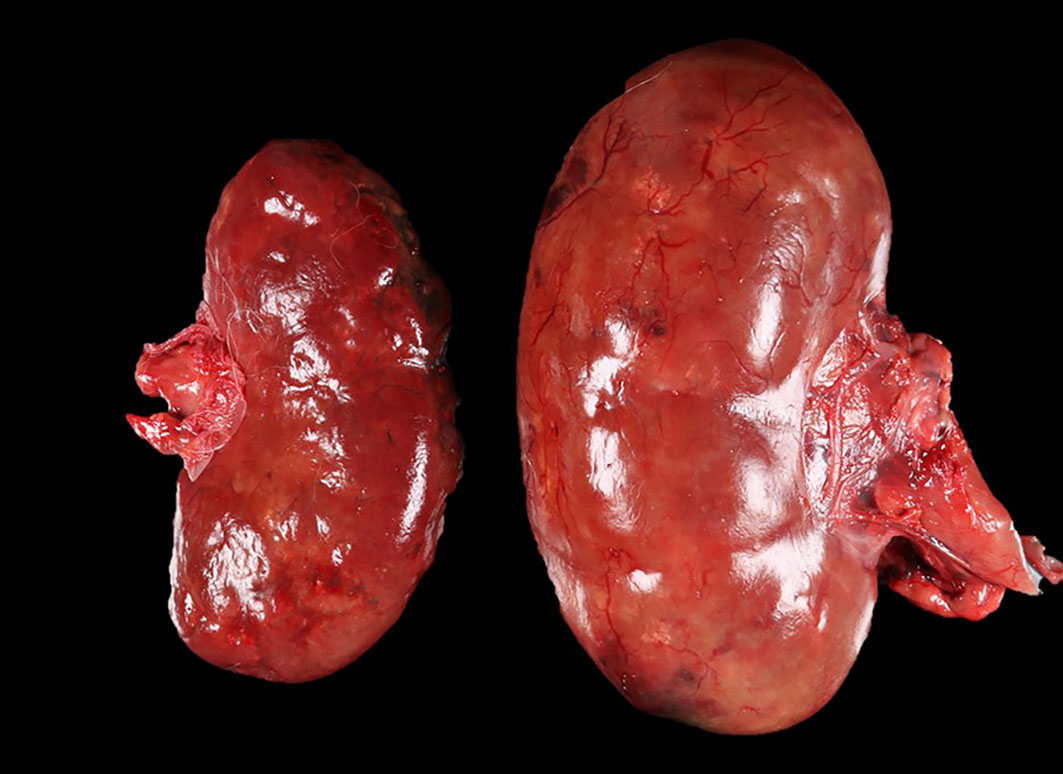
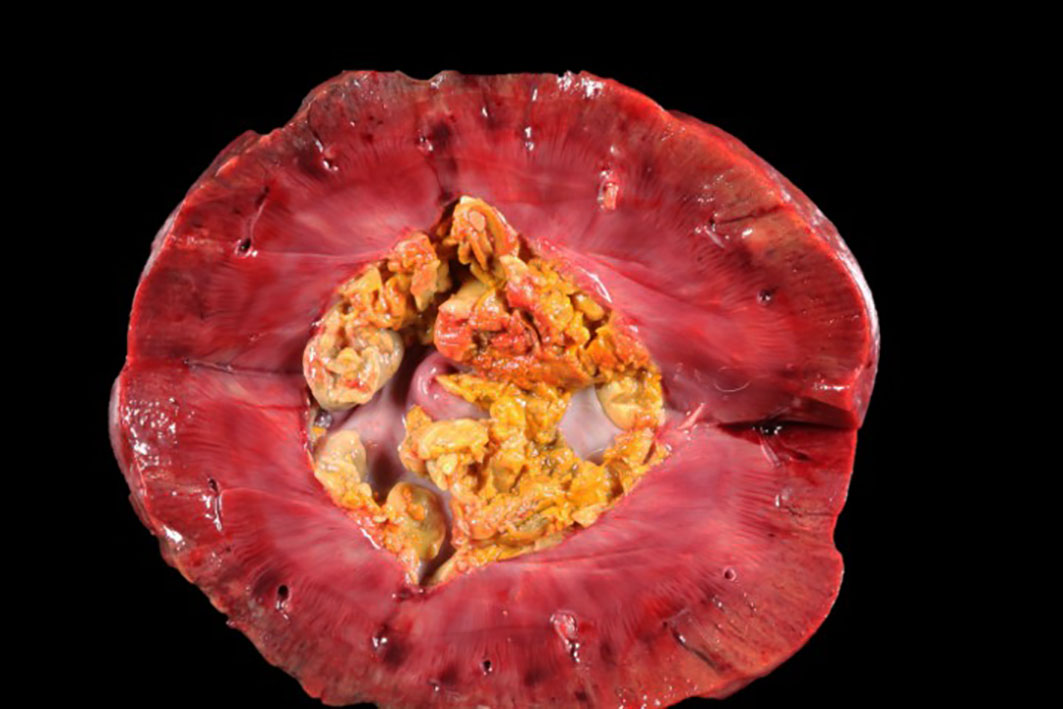
The patient was in a fresh state of preservation and poor body condition (BCS 2/9; total body weight 19kg), with generalised muscular atrophy and severe dehydration. Gross examination revealed enlargement of the right kidney with multifocal 4-6mm white plaques on the cortical surface interspersed with multifocal, variably sized areas of hemorrhage. There was severe bilateral pyelonephritis, with pyonephrosis of the right kidney. Multiple raised, pale, variably sized nodules were present in the splenic parenchyma and in two mesenteric lymph nodes. There was discospondylitis of the lumbar spine at the L3-L4 junction, and osteomyelitis of the cervical spine at the C4-C5 region. The lungs appeared normal and no other abnormalities were noted.
Laboratory Results:
Rasamsonia (Geosmithia) argillacea was cultured from urine and blood.
Microscopic Description:
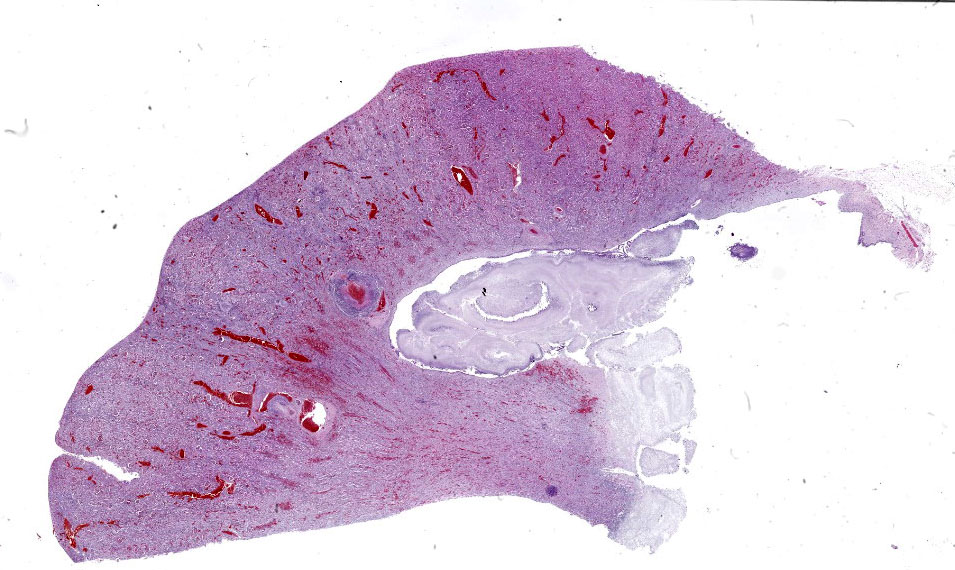
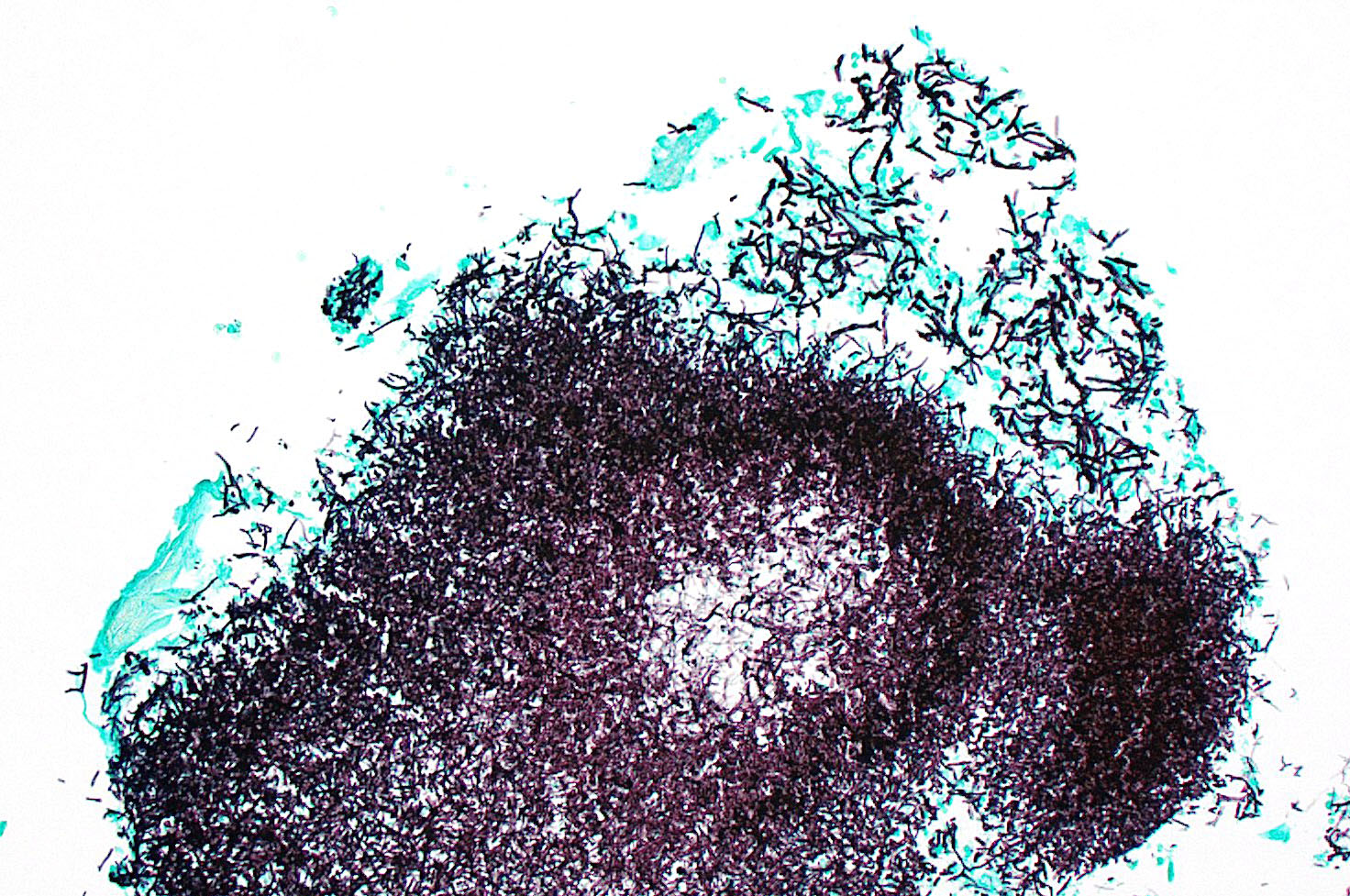
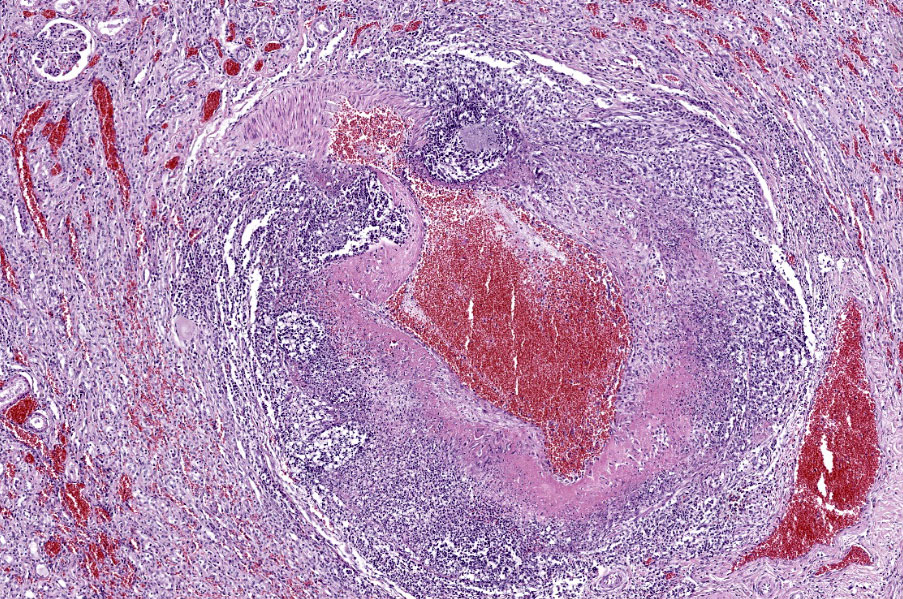
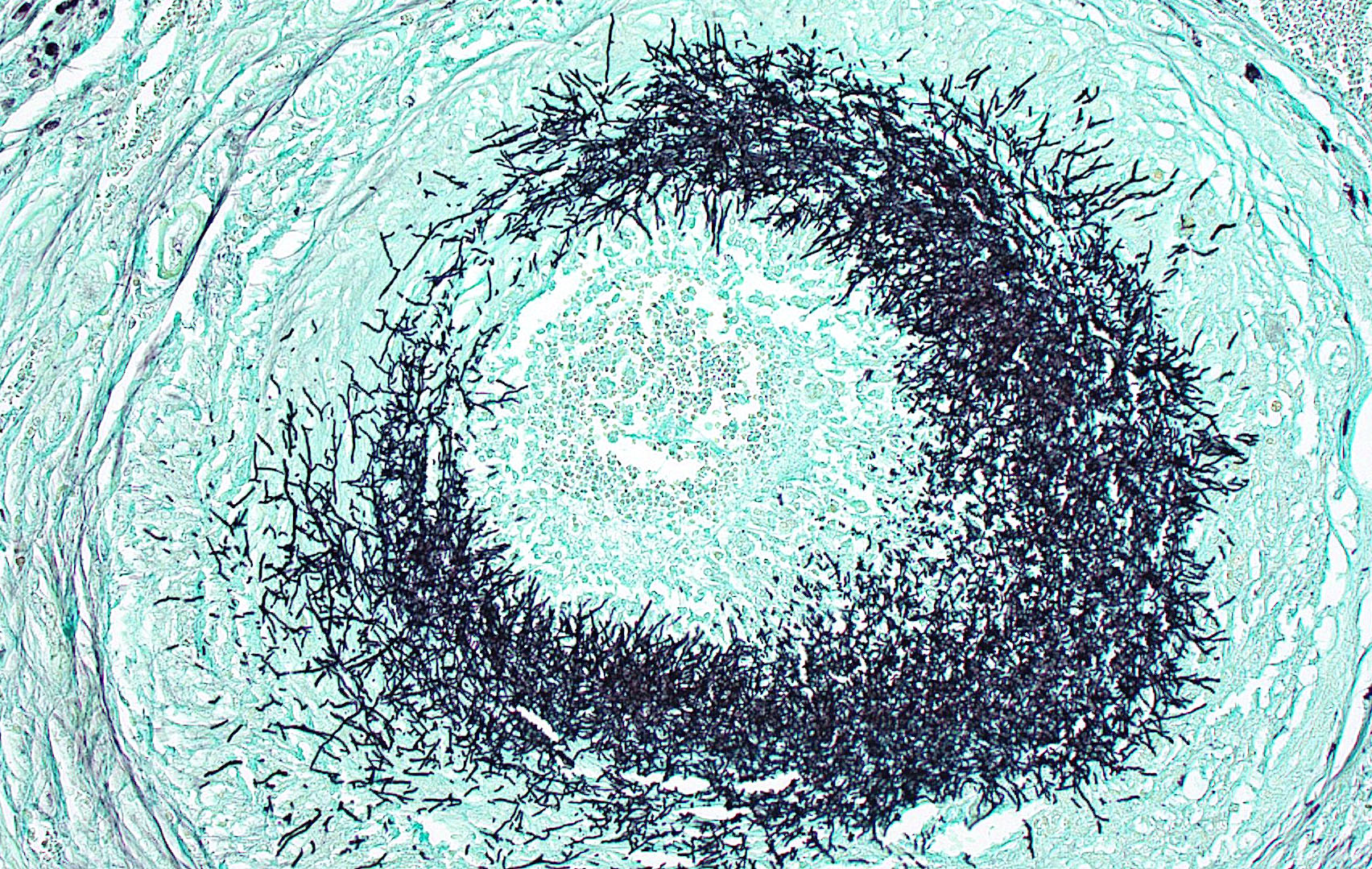
Kidney: Multifocally throughout the cortex, glomeruli and tubules are surrounded and replaced by numerous fibroblasts and abundant eosinophilic collagen bundles (interstitial fibrosis). Tubular epithelial changes include marked epithelial degeneration and necrosis characterized by swollen, vacuolated epithelial cells and shrunken hypereosinophilic, pyknotic cells, respectively. Deeply basophilic granular material (mineral), or bright eosinophilic homogenous material (protein) is present within the lumen of many tubules. Multifocally within the cortex are large aggregates of macrophages, lymphocytes, and plasma cells which surround large regions of amorphous eosinophilic material (fibrin), cellular debris, and 5-10µm diameter, fine, parallel walled, occasionally branching, septate hyphae and 5-7µm diameter conidia. These frequently appear adjacent to arcuate arteries and veins, with numerous PAS positive hyphae disrupting and invading the endothelium (angioinvasion) with marked endothelial necrosis characterized by shrunken, hypereosinophilic pyknotic cells, and destruction of the adventitia and tunica media. Within the renal pelvis, a large area of amphophilic necrotic debris is admixed with abundant PAS positive fungal hyphae and conidia. Multifocally throughout the cortex and medulla there are interstitial aggregates of lymphocytes and plasma cells.
Similar granulomatous inflammation, with PAS-positive fungal hyphae and conidia, is present within the mesenteric lymph nodes, spleen, and lumbar vertebral segment (not submitted).
Contributor’s Morphologic Diagnoses:
- Kidney: Nephritis, interstitial, granulomatous, chronic, multifocal, severe, with numerous PAS positive fungal hyphae and conidia, angioinvasion and marked interstitial fibrosis with cortical tubular degeneration and necrosis, acute, multifocal, marked.
- Kidney: Pyelonephritis, chronic, granulomatous, with numerous PAS positive fungal hyphae and conidia.
Contributor’s Comment:
Here we present a case of disseminated systemic fungal infection with Rasamsonia argillacea. Fungal pyelonephritis with systemic dissemination is an uncommon clinical presentation in dogs, with the most common fungal isolate in canine pyelonephritis being Aspergillus spp., with A. terreus and A. reflectus the most frequently reported species.2,4,8 Cryptococcus spp., Candida spp., Penicillium spp., Paecilomyces spp., Sagenomella spp., as well as systemic phaeohyphomycosis, are less commonly reported.1,5
In dogs, disseminated mycoses can clinically manifest as weight loss, lethargy, discospondylitis, osteomyelitis, urinary tract infections, polyuria, polydipsia, ophthalmitis, head tilt and gait difficulties.4
Rasamsonia is a recently described genus of saprobic thermotolerant fungal organisms of the Trichocomaceae family.3 These are nonpigmented filamentous fungi and include four species that share phenotypic and genetic similarities: R. argillacea, R. eburnean, R. piperina, and R. aegroticola.3 These fungi were previously classified as Geosmithia and Talaromyces, however recent studies reclassified the fungus as Rasamsonia based on genetic sequencing.6,7
The first case of disseminated Rasamsonia (referred to as Geosmithia argillacea) in humans or animals was reported in a German Shepherd.5 German Shepherds appear overrepresented, perhaps owing to their susceptibility to disseminated fungal disease, particularly with Aspergillus spp.2,9 IgA deficiency has been suggested as a possible predisposing factor for disseminated aspergillosis in this breed.11,10 Other factors that may contribute to the German Shepherd’s susceptibility to systemic fungal mycoses include depressed IgM responses or impaired mitogen-induced lymphocyte transformation.11 In regard to R. argillacea infection, one study reported that five of eight dogs with R. argillacea were German Shepherds and six were spayed females.3
To our knowledge this is the first report of Rasamsonia (Geosmithia) argillacea causing disseminated disease in a dog in New Zealand or Australia. Due to the scant number of reported cases, the pathogenesis of R. argillacea infection is not well established. In most cases of systemic fungal mycoses, the portal of entry is usually unknown.4 The most common portal of entry for fungal infections is the respiratory tract via inhalation, which then allows systemic spread after an initial respiratory infection.4 Based on studies involving Aspergillus spp., the cycle begins with deposition of fungal conidia from the environment in the respiratory system.4 Fungal species such as Paecilomyces spp. have also been reported to produce in vivo conidia, and these, along with the secondary spores produced, facilitate hematogenous spread.4
The portal of entry could not be identified in this case, which is consistent with the reported literature.4 The presenting complaint
of lumbar spinal pain with discospondylitis was the first indication of any clinical disease in this dog. The dog had a prior bout of suspected kennel cough, but this was over 12 months prior to the presentation of lumbar spinal pain, so perhaps this is where the initial infection was acquired. It is also possible that the infection was first acquired via the urinary tract, which then lead to pyelonephritis and systemic spread. Primary fungal pyelonephritis in dogs is only rarely reported.5 In one case report, unilateral pyelonephritis was reported, with no evidence of systemic spread, indicating a possible ascending infection from the urinary tract.5 In another case report, a primary fungal granuloma with Paecilomyces variotii, with no other evidence of systemic involvement was reported.10 In both studies the primary route of entry could not be identified. It has also been suggested that immunosuppression due to other pathological conditions or immunosuppressive therapy may play a role.4
The gross and histological lesions in this case are astounding, with numerous fungal hyphae, granulomatous inflammation, angioinvasion and multinucleated giant cells within multiple organs. Rasamsonia argillacea appears to be an emerging pathogen in both human and animal medicine.3 With the increasing reports in dogs, more research is needed to help further characterize Rasamsonia infections and to optimize diagnosis and treatment to improve overall prognosis.
Contributing Institution:
School of Veterinary Science
Massey University
Palmerston, North New Zealand 4442
JPC Diagnosis:
Kidney: Pyelonephritis, necrotizing and suppurative, focally extensive, severe, with fibrinous and necrotizing arteritis, multiple infarcts, and innumerable fungal hyphae.
JPC Comment:
The contributor provides an excellent overview of Rasamsonia infection in dogs. Though case reports are scarce, recent reports suggest that disseminated Rasamsonia infections commonly cause lesions in the spinal column, central nervous system, kidneys, spleen, lymph nodes, lungs, and heart.3 Rasamsonia is considered an emerging pathogen, though it is frequently misidentified as Penicillium and Paecilomyces spp. based on morphologic similarities, and the apparent increase in incidence may be due, in part, to increased use of more accurate molecular speciation techniques in recent years.
This case is typical, in that the presenting complaint is often spinal pain due to Rasamsonia discospondylitis. Imaging studies of these patients typically reveal sclerotic and lytic vertebral lesions, most commonly in the thoracic spine.3 These radiographic findings are similar to those in the relatively more common condition of disseminated aspergillosis, where approximately half of affected dogs show radiographic signs of discospondylitis.9 Treatment in these cases is generally unsuccessful due to fungal resistance to available antifungal medications and the questionable ability of these medications to penetrate all affected tissues.5
In conference, discussion centered on the acute versus chronic nature of the observed histologic lesions. Large confluent areas of coagulative necrosis are present adjacent to the renal pelvis and in wedges extending from the medulla to the cortex. There are also extensive areas of fibrosis which represent more chronic insults arising from the damage to the arcuate arteries. Discussion also focused on the morphology of the dazzling fungal hyphae present in the renal pelvis and in arterial walls. Participants discussed differentials, including Candida spp. and Penicillium spp., and the need for fungal culture to identify the organism definitively.
References:
- Day M, Holt P. Unilateral fungal pyelonephritis in a dog. Vet Pathol. 1994;31: 250-252.
- Day M, Penhale W. An immunohistochemical study of canine disseminated aspergillosis. Aus Vet J. 1991;68:383-386.
- Dear JD, Reagan KL, Hulsebosch SE, et al. Disseminated Rasamsonia argillacea species complex infections in 8 dogs. J of Vet Int Med. 2021;35:2232-2240.
- Elad D. Disseminated canine mold infections. Vet J. 2019;243:82-90.
- Grant DC, Sutton DA, Sandberg CA, et al. Disseminated Geosmithia argillacea infection in a German shepherd dog. Med Mycol. 2009;47:221-226.
- Houbraken J, Giraud S, Meijer M, et al. Taxonomy and antifungal susceptibility of clinically important Rasamsonia J of Clin Microbiol. 2013;51:22-30.
- Houbraken J, Spierenburg H, Frisvad JC. Rasamsonia, a new genus comprising thermotolerant and thermophilic Talaromyces and Geosmithia Antonie Van Leeuwenhoek. 2012;101:403-421.
- Jang S, Dorr T, Biberstein E, Wong A. Aspergillus deflectus infection in four dogs. J of Med and Vet Mycol. 1986;24: 95-104.
- Schultz RM, Johnson EG, Wisner ER, et al. Clinicopathologic and diagnostic imaging characteristics of systemic aspergillosis in 30 dogs. J of Vet Int Med. 2008;22:851-859.
- Tappin S, Ferrandis I, Jakovljevic S, Villiers E, White R. Successful treatment of bilateral paecilomyces pyelonephritis in a German shepherd dog. J of Small Ani Prac. 2012;53:657-660.
- Zhang S, Corapi W, Quist E, Griffin S, Zhang M. Aspergillus versicolor, a new causative agent of canine disseminated aspergillosis. J of Clin Microbiol. 2012;50: 187-191.