Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 14
9 January, 2019
CASE III: S1603556 (JPC 4084299).
Signalment: A 14-month-old, 5 kg, female, Nigerian dwarf goat (Capra hircus)
History: A total of 4 goats in an original herd of 8, died in a period of 8 months with a history of limping, progressive loss of body condition, and difficulty in getting up. Three goats died naturally and one was euthanized. This goat died naturally.
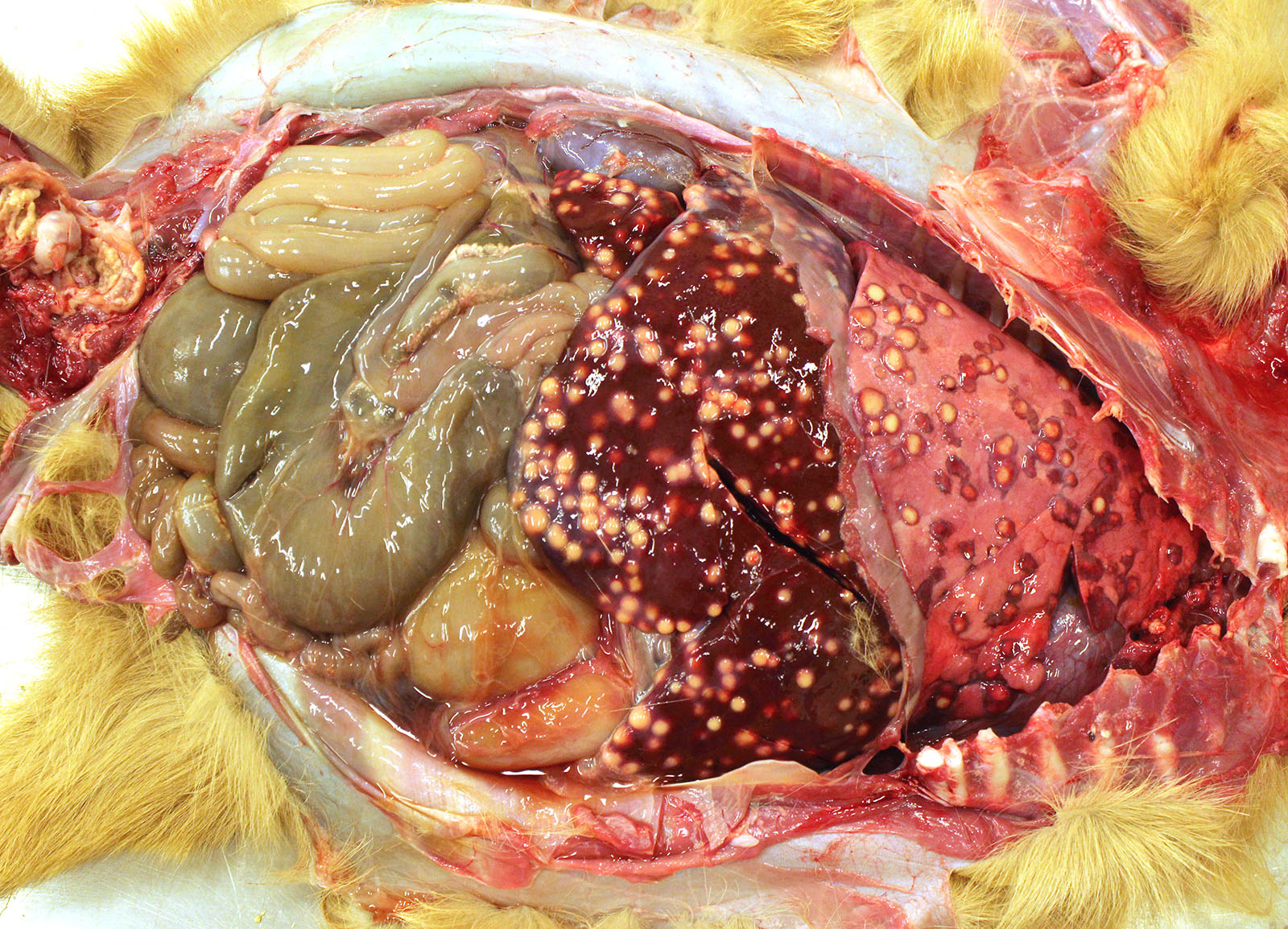
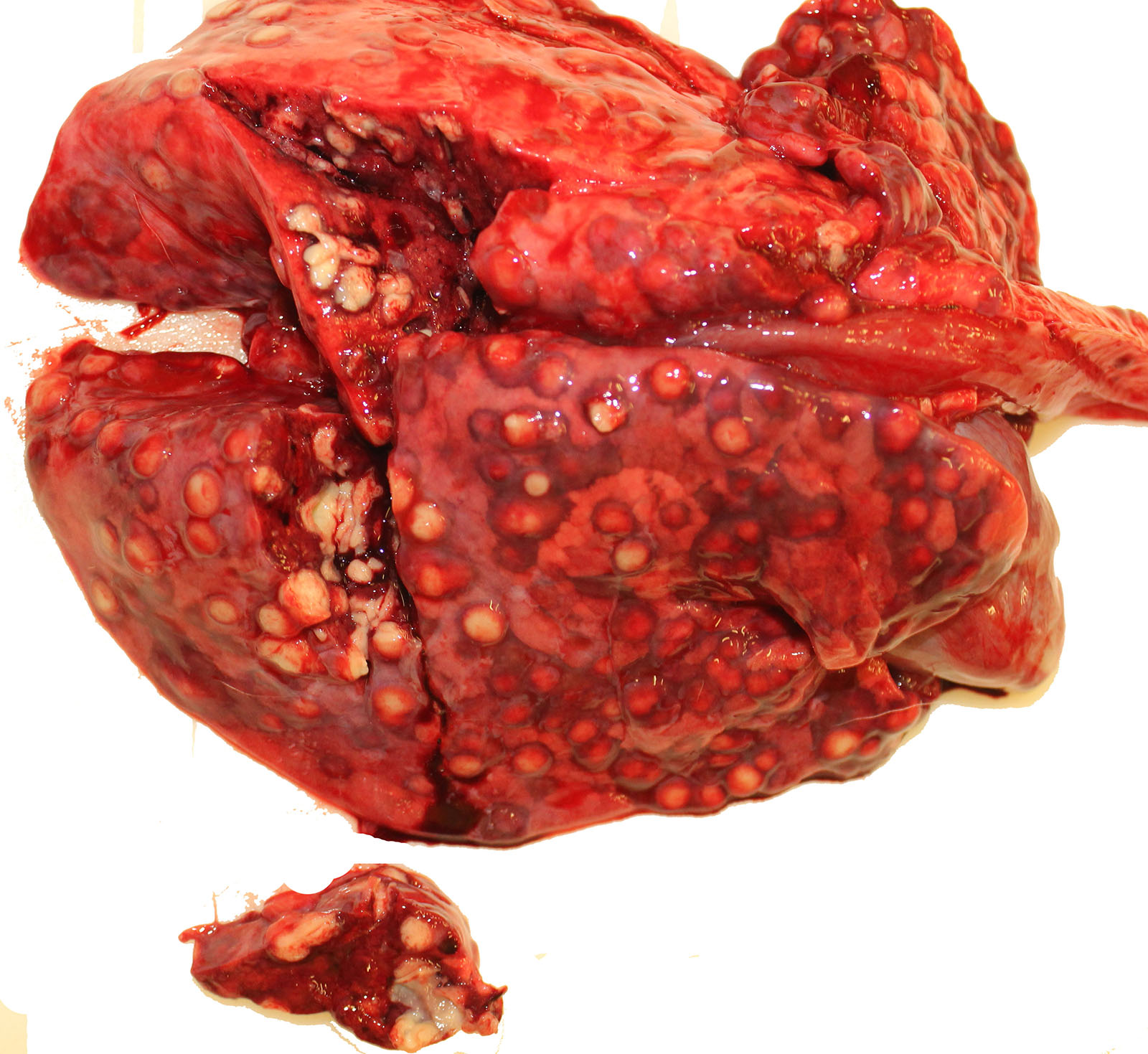
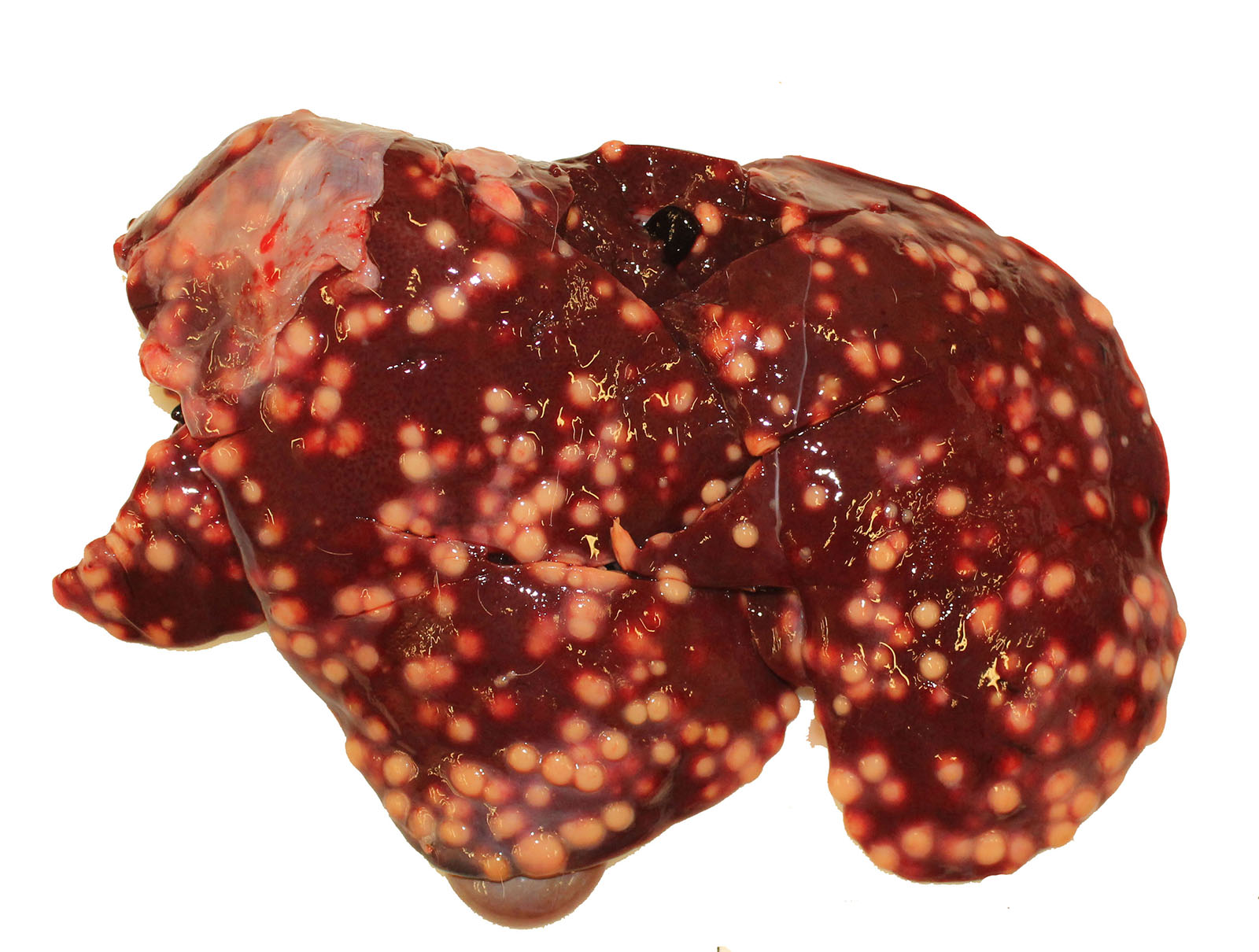
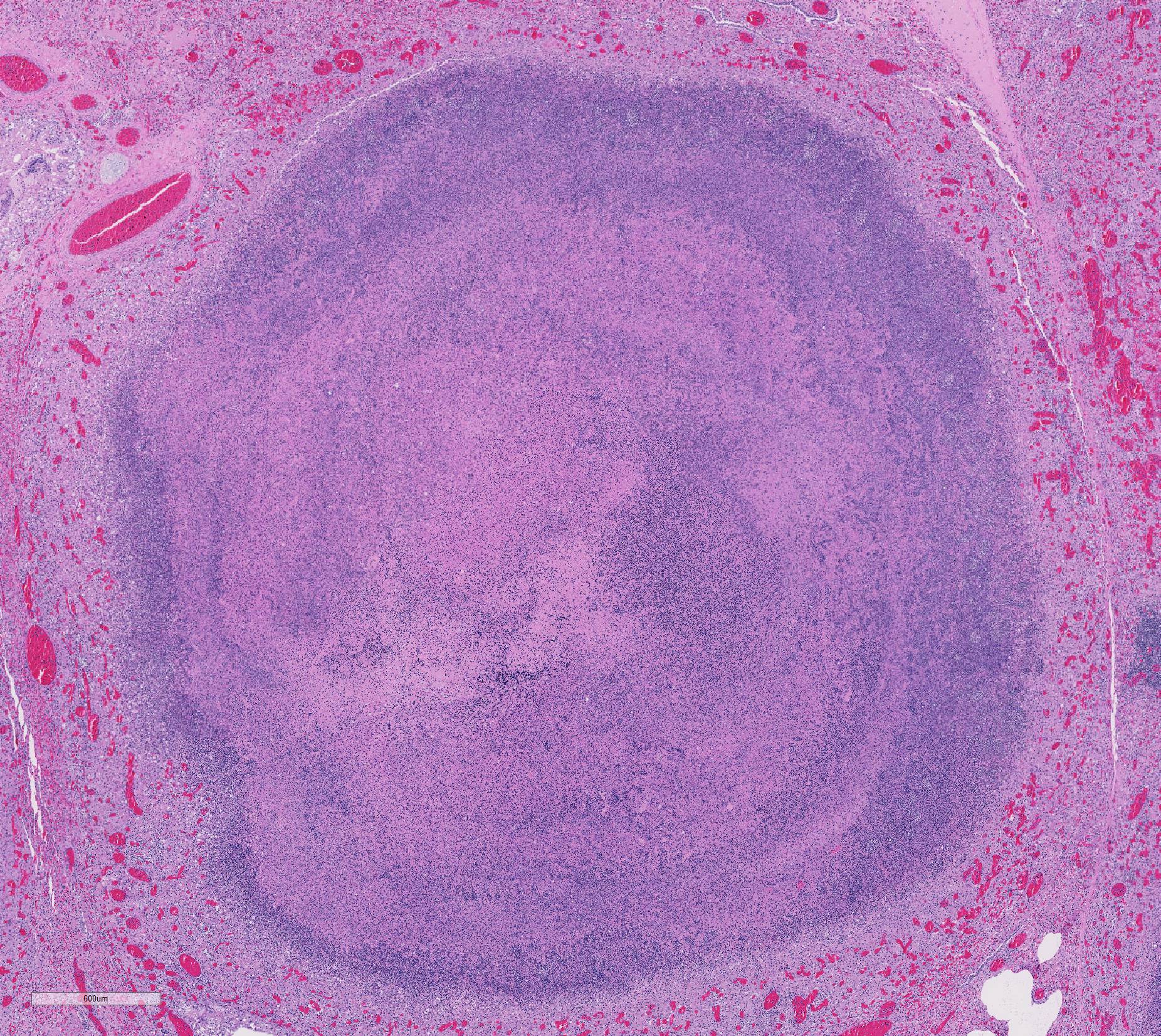
Gross Pathology: The thoracic cavity contained approximately 25 mL of thin, pink fluid. The lungs and liver had innumerable disseminated, soft to semi-firm, pale yellow, 0.3-0.6 cm diameter, occasionally raised nodular foci scattered throughout the parenchyma, which frequently oozed pale yellow, thick liquid upon sectioning. The spleen had a few similar foci.
Laboratory results: Bacteriology: aerobic bacterial culture of the liver yielded heavy growth of Rhodococcus equi, Streptococcus sp. and Staphylococcus sp
Microscopic Description:
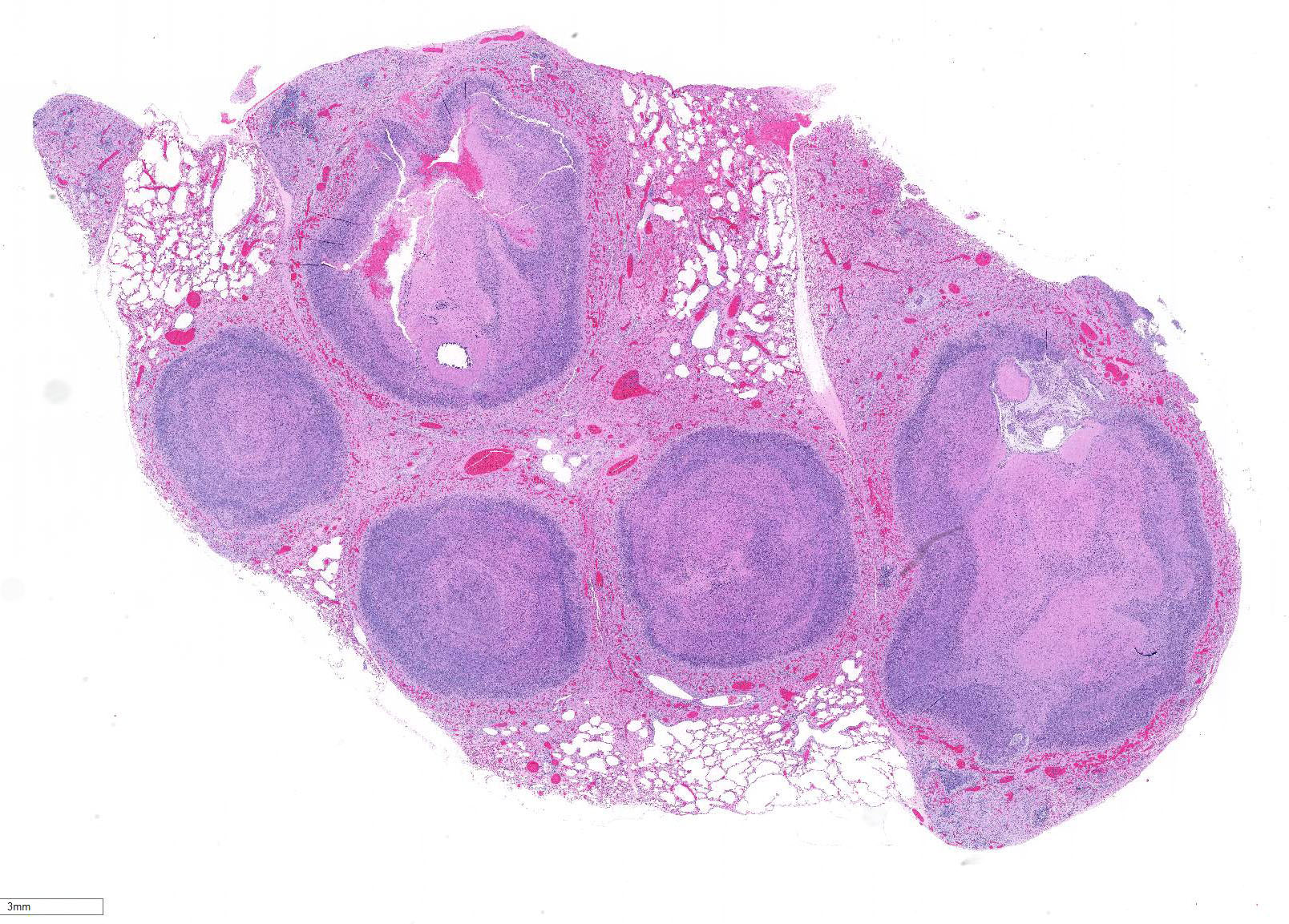
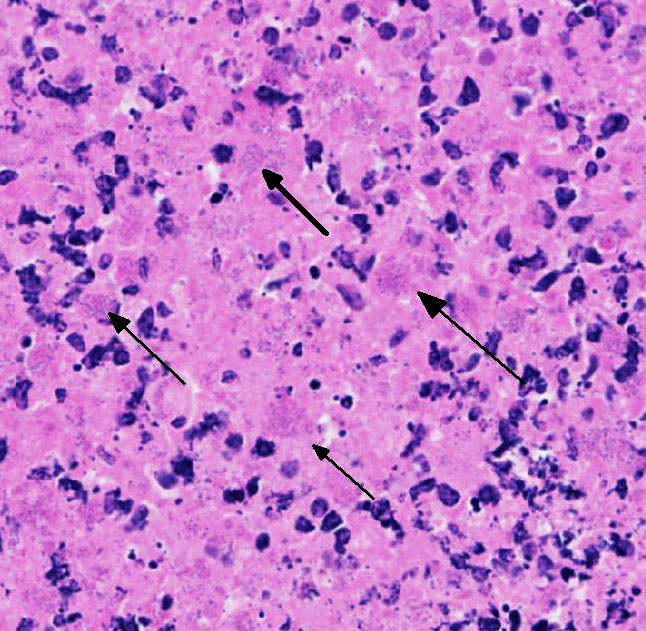
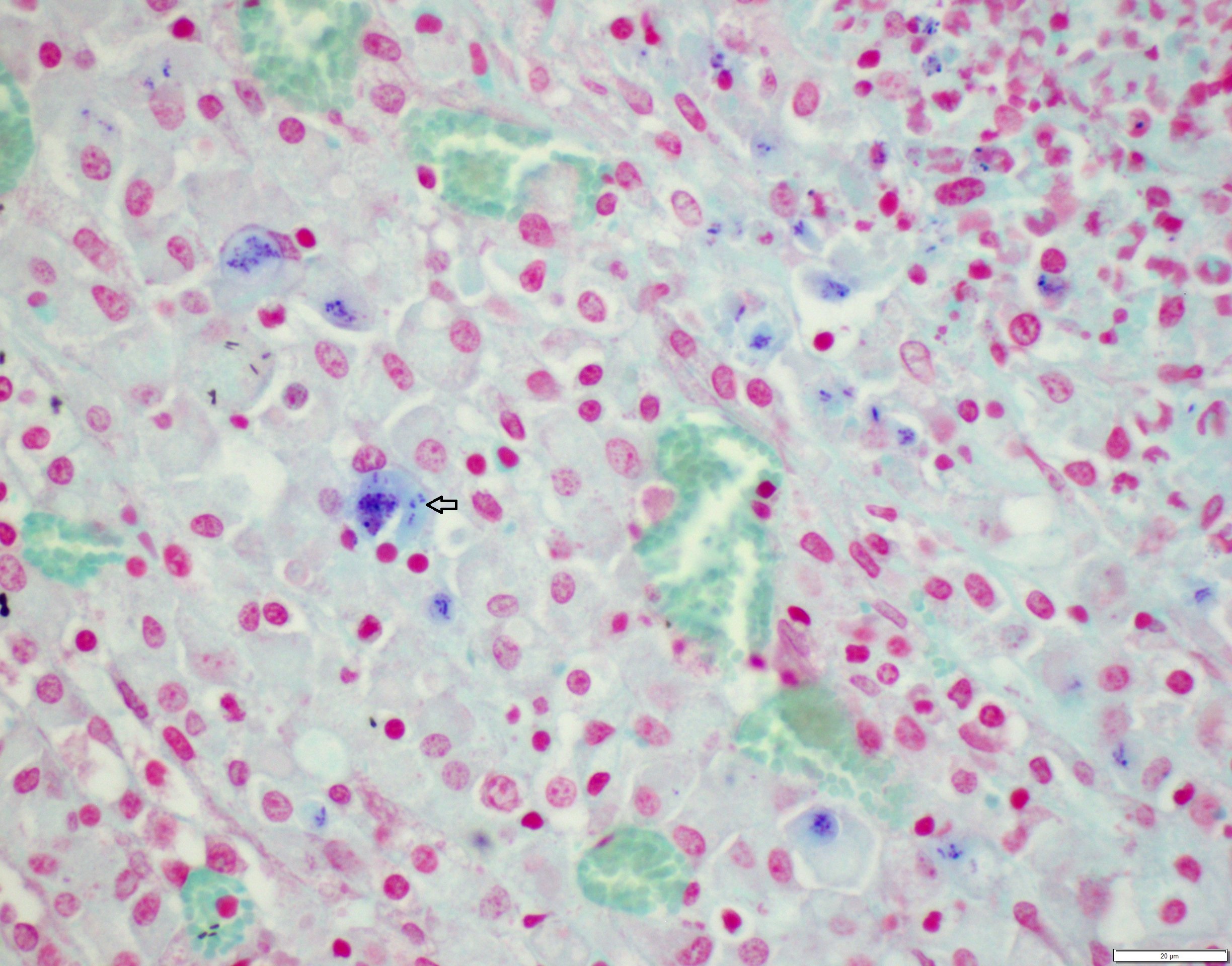
Lung: centering in small caliber vessels and extending into the adjacent alveolar spaces and septa, there is a multifocal and random inflammatory infiltrate composed by large numbers of viable and degenerated neutrophils, fewer histiocytes and occasional multinucleated giant cells that are admixed with basophilic cellular debris and several rounded eosinophilic protozoa of approximately 15 to 20 µm in diameter, with an eccentric single round nucleus. In addition, there are numerous fibrin thrombi and segments of alveolar necrosis. A few blood vessels display transmural necrosis, with fibrinoid degeneration and the presence of pyknotic debris. The remaining parenchyma shows moderate congestion, occasional small hemorrhages and multiple focal areas of intra-alveolar edema.
Contributor’s Morphologic Diagnosis:
Lung: marked, multifocal to coalescing, pyogranulomatous, pneumonia with myriad intrahistiocytic, gram-positive, coccobacillary bacteria
Other tissues (Slides not submitted):
Liver: marked, multifocal to coalescing, pyogranulomatous, hepatitis with intrahistiocytic, gram-positive, coccobacillary bacteria
Spleen: 1. mild, multifocal, nodular, pyogranulomatous, splenitis with multifocal mineral deposition, intrahistiocytic, gram-positive, coccobacillary bacteria; 2. marked histiocytosis
Skeletal muscle: marked, focally extensive, pyogranulomatous, myositis with myriad intra-histiocytic coccobacillary bacteria
Contributor’s Comment: The morphology and distribution of the lesions found in this goat were consistent with a disseminated rhodococcal infection. In goats, this is a recognized cause of disseminated pulmonary and hepatic abscesses2, 4. Rhodococcus equi is classified largely as a soil organism, but has been isolated from the feces of birds and grazing herbivores. It has been suggested that R. equi may be widespread in herbivores and their environment because their manure supplies the substrates on which the organism thrives5. Inhalation or ingestion of the bacteria is thought to be the major mode of transmission, causing the development of respiratory or enteric infections, respectively8. Subsequent bacteremia and hematogenous dissemination within macrophages to other sites in the body then occurs 8.
Rhodococcus equi is a facultative intracellular gram-positive bacterium which proliferates in macrophages and multinucleated giant cells8. R. equi causes two primary forms of disease in foals which are pyogranulomatous bronchopneumonia and ulcerative enterocolitis. In addition, ulcerative typhlitis with suppurative or granulomatous lymphadenitis, osteomyelitis, synovitis, septic arthritis, and rarely abortion are reported in horses with R. equi infection6, 8. R. equi has also been reported to cause pneumonia in immunosuppressed people, especially in AIDS patients3. R. equi infections are rarely reported in cattle, sheep, pigs, cats, dogs and camelids1, 8. In goats, R. equi typically causes pyogranulomatous lesions in the liver and lungs2, 4. Occasionally, osteomyelitis of the vertebra and skull and fibrinous enterocolitis have also been reported in goats4.
The virulent forms of R. equi contain a plasmid containing a vapA gene (virulence associated protein A) which encodes a 15-17 kDa cell surface lipid protein. This protein can elicit an intense humoral response7, 8. The bacterium is able to prevent phagosome-lysosome fusion by inhibiting opsonization or activation of macrophages by IFN-gamma resulting in bacterial survival within the macrophage, therefore, an intense Th-1 response is required to clear an established infection8.
Contributing Institution:
University of Connecticut
Connecticut Veterinary Medical Diagnostic Laboratory,
Department of Pathobiology and Veterinary Science
College of Agriculture, Health and Natural Resources
http://patho.uconn.edu/
JPC Diagnosis: Lung: Pyogranulomas, multiple, with numerous intrahistiocytic coccobacilli.
JPC Comment: R. equi’s virulence-associated proteins (vapA in virulent strains, and vapB in intermediately virulent strains) are encoded on circular and linear plasmids. Strains that do not contain these proteins are classified as avirulent. Recently, a novel R. equi host-adapted linear virulence plasmid, pVAPN was characterized in cattle isolates, which encodes another virulence –associated protein (vapN). VapG, another protein identified in virulent strains has recently been identified as a potential vaccinal protein which has shown efficacy in the partial protection of R. equi in mice (in addition to current vaccinal work with vapA). In addition, a highly conserved conjugal transfer protein gene (traA) is common to all strains which carry these plasmids and may now be used to identify pathogenic strains.1 In previous studies, the virulent and intermediately virulent strains are primarily identified in foals and pigs, while most reports of R. equi isolated from cattle and dogs appear to be of avirulent (non-vapA, non-vapB) strains.1
A recent article by Bryan et al1 describes the clinical syndrome associated with R equi infection in five dogs. Two of the isolated strains were avirulent, one was vap-A positive, and one carried the vapN virulence-associated plasmid. Four dogs were on immunosuppressive drugs or had endocrinopathies. The varied presentation and distribution of disease in the five dogs reflects the systemic and almost random nature of R. equi infection of the dog, with granulomatous dermatitis , aortic vegetative valvular endocarditis, pulmonary abscessation, and lymphadenitis the most common presentation, but each only being present in two out of five cases.
Considered an emerging pathogen in humans, over 100 cases of R. equi infection have been reported since the first description of its occurrence in 1967, likely as a result of improvement in isolation techniques and recognition of this bacterium as a human pathogen.9 The vast majority of cases have occurred in immunosuppressed patients with over 66% in cases in AIDS patients, and an additional 10% in transplant recipients as a late complication (mean 49 months post transplant). of immunomodulation. 80% of cases involved pulmonary infection, most commonly abscesses with necrotic centers, although pulmonary disease characterized by microabscesses is also common. As in other species, pyogranulomatous infection may occur in a wide range of organs. Treatment involves regimes of multiple antibiotics (often including rifampin) and is complicated by the typical presence of immunosuppression in affected individuals.9
The moderator reviewed the differences between Corynebacterium pseudotuberculosis and Rhodococcus equi. Additionally, he identified several recent review articles discussing rhodococcal infection in several atypical species such as dogs1 and goats2,4.
References:
- Bryan LK, Clark SD, Diaz-Delgado J. Rhodococcus equi Infections in Dogs. Vet Pathol. 2017; 54(1):159-163.
- Davis WP, Steficek BA, Watson GL. Disseminated Rhodococcus equi infection in two goats. Vet Pathol. 1999;36(4):336-339.
- Ferretti F, Boschini A, Iabichino C. Disseminated Rhodococcus equi infection in HIV infection despite highly active antiretroviral therapy. BMC Infect Dis 2011; 11:343-2334-11-343.
- Jeckel S, Holmes P, King S. Disseminated Rhodococcus equi infection in goats in the UK. Vet Rec 2011; 169(2):56.
- Muscatello G. Rhodococcus equi pneumonia in the foal--part 1: pathogenesis and epidemiology. Vet J 2012; 192(1):20-26.
- Szeredi L, Molnar T, Glavits R. Two cases of equine abortion caused by Rhodococcus equi. Vet Pathol 2006; 43(2):208-211.
- Trevisani MM, Hanna ES, Oliveira AF, et al. Vaccination of Mice with Virulence-Associated Protein G (VapG) Antigen Confers Partial Protection against Rhodococcus equi Infection through Induced Humoral Immunity. Front Microbiol 2017; 8:857.
- Vazquez-Boland JA, Giguere S, Hapeshi A, et al. Rhodococcus equi: the many facets of a pathogenic actinomycete. Vet Microbiol 2013; 167(1-2):9-33.
- Weinstock DM, Brown AE. Rhodococcus equi: an emerging pathogen. Clin Inf Dis 2002: 34:1379-85.