Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 6
2 October 2019
Dr. Jeff Wolf, DVM
Senior Pathologist
Chief Scientific Officer/Pathology Manager
EPL, Inc.
Sterling, VA
CASE I: A15-62176 (JPC 4118173).
Signalment: Adult (age unknown), female, cardinal tetra (Paracheirodon axelrodi) fish
History: Following transport by air, high mortalities began in the group of fish approximately 24-hours after arrival in quarantine facilities at a public aquarium. There were no reported gross lesions. Skins scrapes, gill, and fin clips were reported as negative for external parasites.
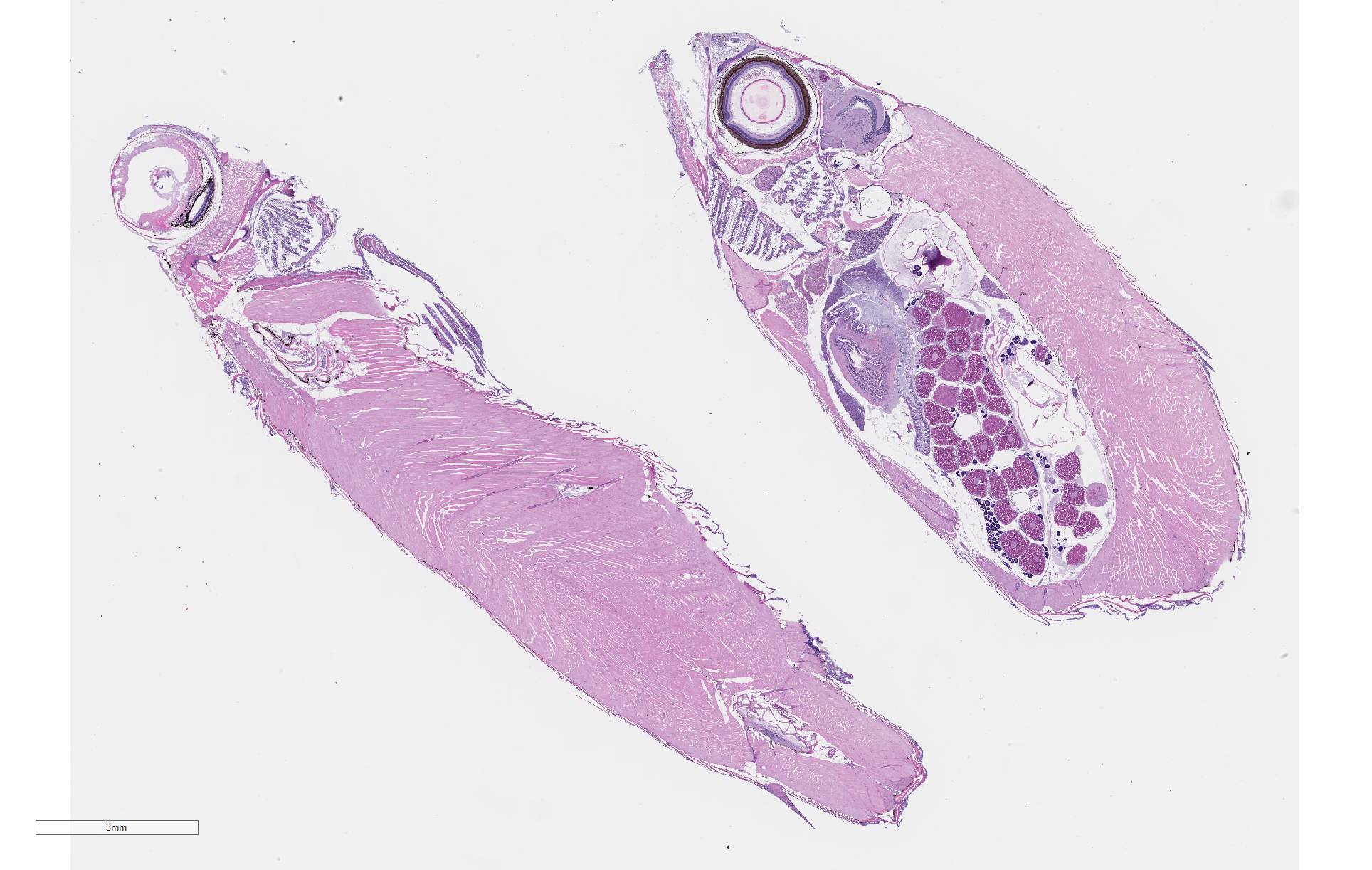
Gross Pathology: Four fish were received whole fixed in formalin. Skin surfaces contained multiple, ill-defined areas of pallor and scale loss suggestive of erosion or ulceration.
Laboratory results: N/A
Microscopic Description:
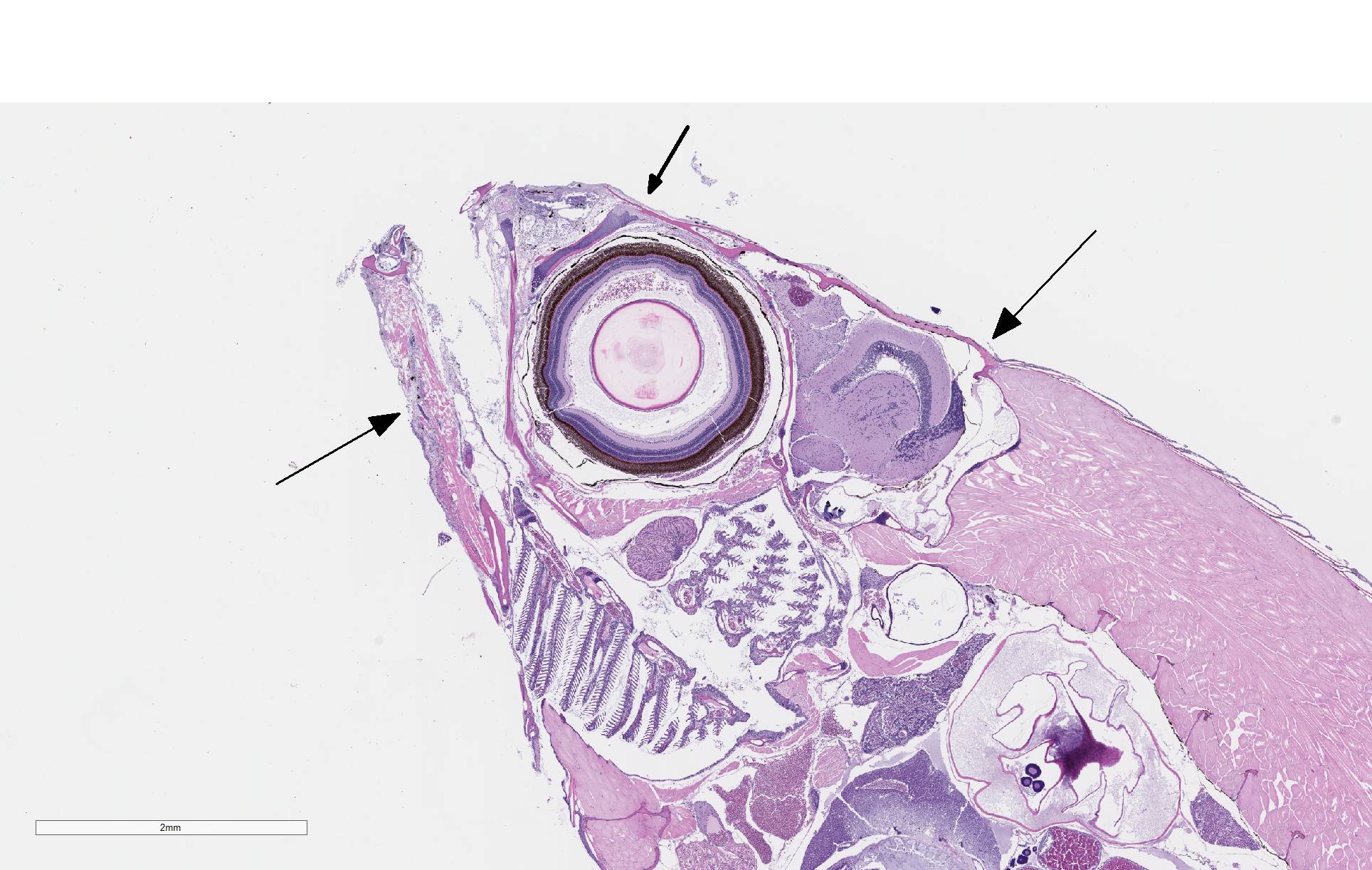
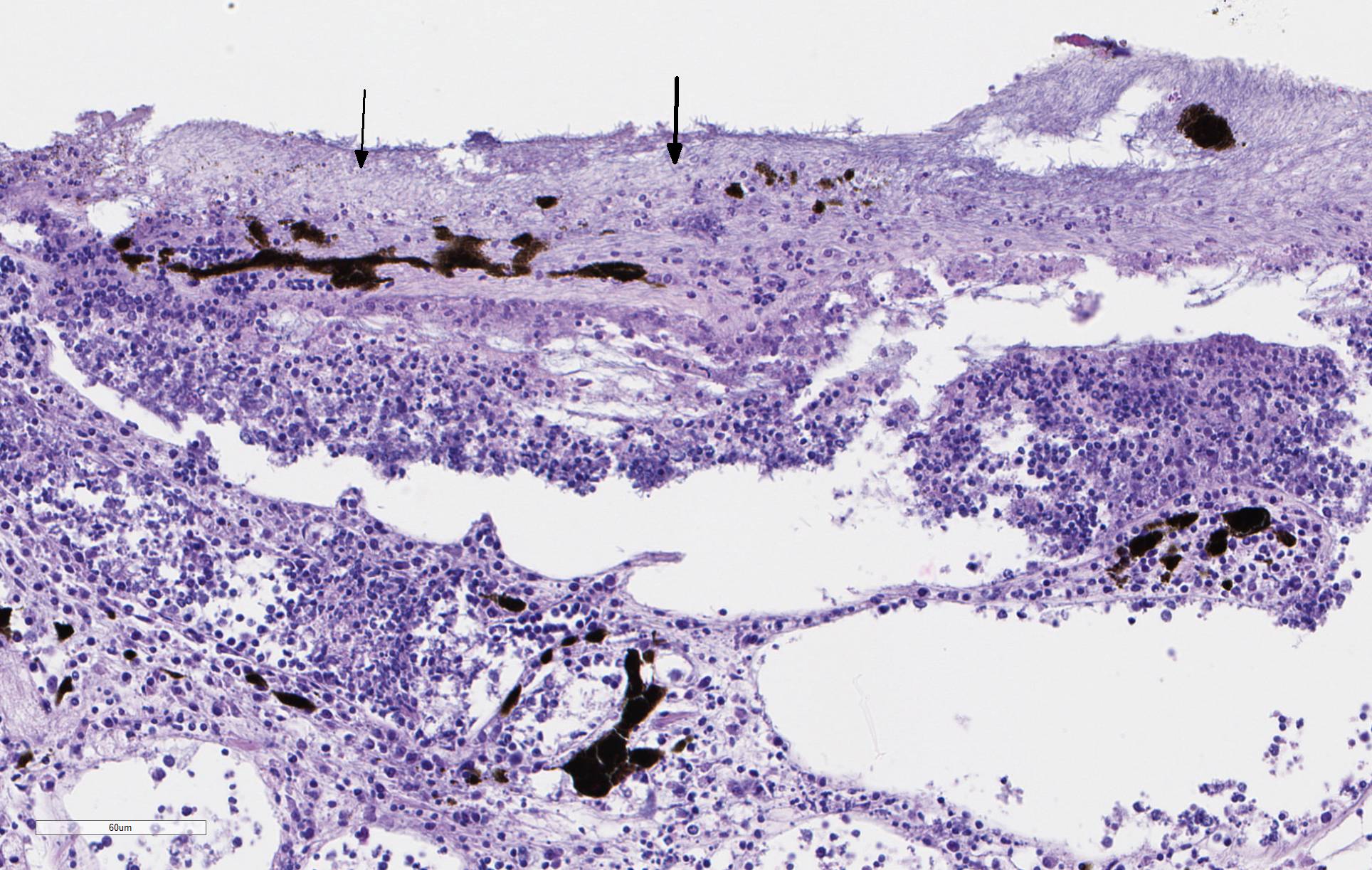
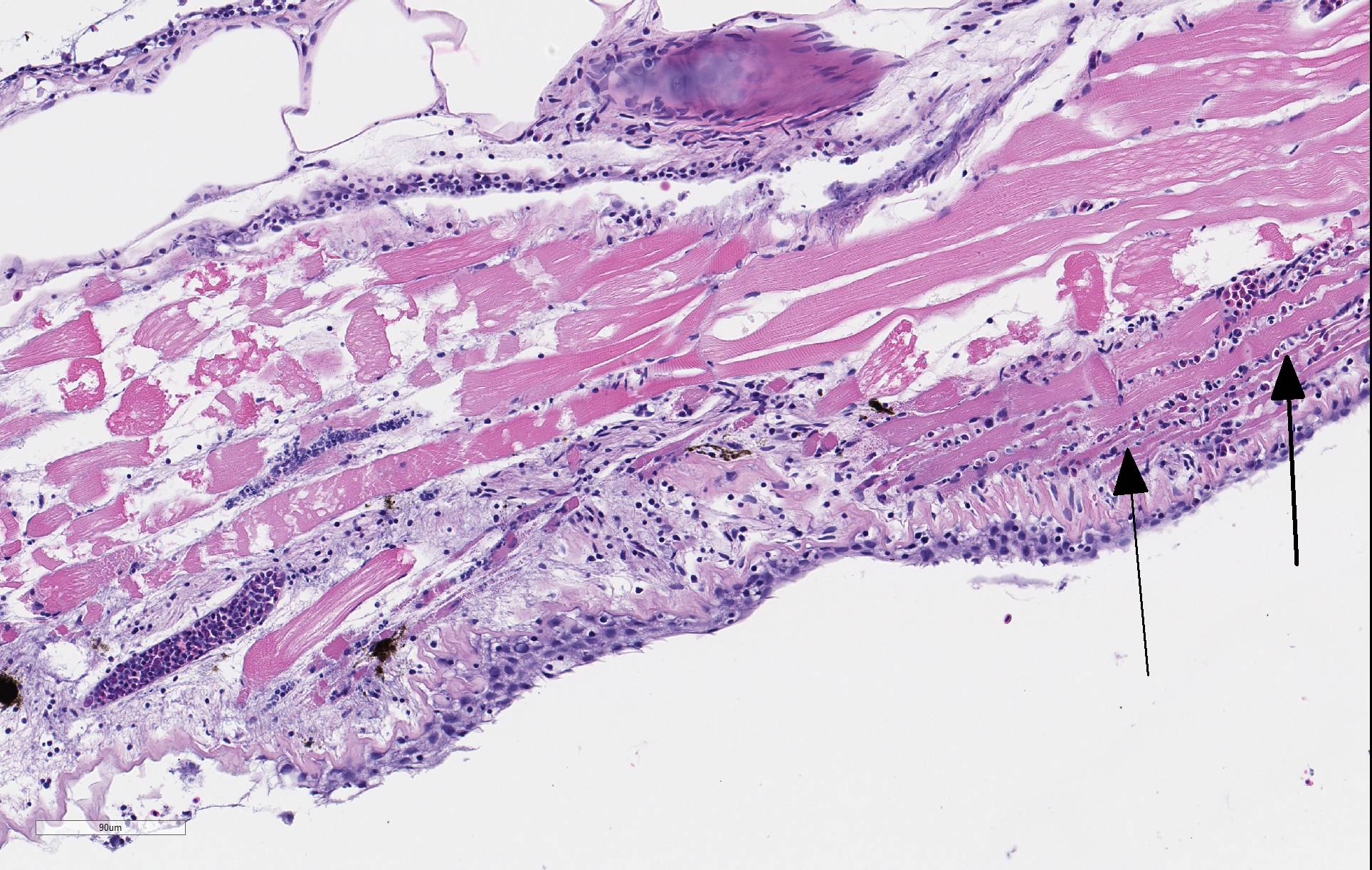
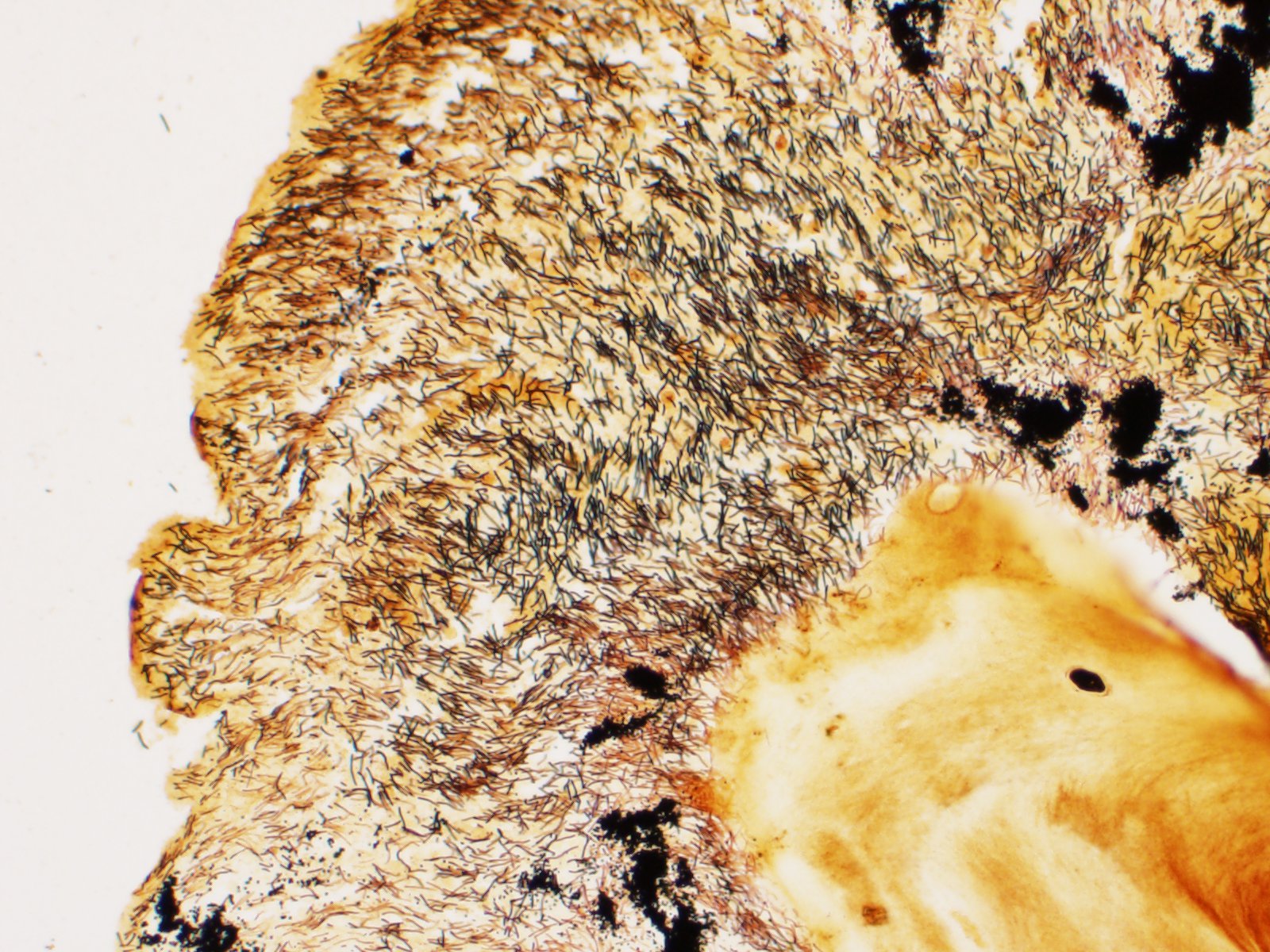
There is severe ulceration of the dorsal cranial region, snout, and lips, extending onto the ventral mandibular region and into the oropharynx. Lesions are characterized by extensive epithelial loss, with variable loss or necrosis of dermal connective tissues. Remaining dermal tissues are pale staining, have lost morphologic detail and are infiltrated by masses of a monomorphic population of slender, approximately 4-6 um long, bacterial rods. Necrosis and edema extend into underling muscle and adjacent soft tissues, including the olfactory rosette. Affected myofibers are similarly pale staining, with coagulated, vacuolated and fragmented cytoplasm, devoid of cross striations. Associated hypodermal adipose and areolar connective tissues are edematous, contain abundant cellular debris, and have lost tinctorial properties and morphologic detail. Bacteria are scattered throughout. Inflammatory responses vary from none to infiltration by small to moderate mixed populations of lymphocytes and macrophages. A focal area of hemorrhage extends from the forebrain into the cranial cavity.
Contributor Morphologic Diagnosis:
Facial skin: Ulcerative dermatitis, necrotizing, acute, focally extensive, severe, with slender bacterial rods, and subadjacent necrotizing myositis, cellulitis, and olfactory mucosal necrosis
Contributor Comment: Microscopic examination reveals ulcerative skin lesions containing large numbers of long, slender bacterial rods consistent with Flavobacterium columnare, the causative agent of columnaris disease. Similar ulcerative lesions were present in all fish examined, varying only in lesion distribution and severity. Additional findings included the presence of several encapsulated larval nematodes in the coelomic cavity.
Columnaris disease occurs worldwide in many wild and cultured freshwater fish, including tropical aquarium species.3 In the southeastern United States it is a major pathogen affecting channel catfish aquaculture.4 The term "saddle back" is commonly used to describe symmetrical lesions over the dorsum of the back, but this is only one manifestation of the disease, which frequently involves various combinations of the gills, perioral area, fins, and caudal peduncle. The clinical course of disease, acute to chronic, and extent of lesions is dependent on the virulence of the bacterial strain involved.3 Lesions generally begin as foci of depigmentation and erosion, but can rapidly progress to large ulcers with exposure of underlying skeletal muscle. Lesions often have a yellowish discoloration and narrow hemorrhagic border. Microscopically, lesions are primarily necrotizing, often in the presence of massive numbers of the long slender bacteria, particularly within dermal connective tissues. Bacteria are readily visualized in H&E-and Giemsa-stained sections. Despite the large numbers of bacteria and their destructive nature in tissue, inflammatory cell infiltration is often minimal. Secondary infection by the oomycete Saprolegnia sp. is common.3,4
Flavobacterium columnare is widespread in freshwater environments and survival is promoted in hard alkaline water with high organic loads. While highly virulent strains of F. columnare exist, the bacteria often presents as a typical opportunist following episodes of environmental stress, such as transport, as seen in this case. Other environmental factors conducive to infection include higher temperatures, high stocking densities, elevated nitrite levels, and slow water flow. Disease pathogenesis is poorly understood, but may initially involve factors related to the ability of the bacteria to respond to chemotaxic factors, adhere to host surfaces, and aggregate in thick mats. Three genomovars of F. columnare with variable pathogenicity are known to exist. Pathogenicity is enhanced by the presence of a thick capsule and the production of chondroitin AC lyase, which degrades chondroitin sulfates and hyaluronic acid in connective tissues. Extracellular proteases also contribute to tissue damage and promote invasion. Some studies indicate impairment of the host alternative complement pathway through sialic acid production and it is hypothesized that lack of inflammation may be related to the production of pro-apoptotic factors that inhibit phagocytes. Reported disturbances in blood parameters, presumably the result of water imbibition, include decreases in PCV, electrolytes and serum proteins.3
The name @quot;columnaris disease" has been used consistently since the condition was first described in 1922. However, the taxonomic status of the agent has been revised numerous times and includes the earlier combinations Bacillus columnaris, Chondrococcus columnaris, Cytophaga columnaris, and Flexibacter columnaris.3 The current name, Flavobacterium columnare, was recognized in 1996.2 The genus Flavobacterium contains additional important fish pathogens that can produce lesions similar to F. columnare, including Flavobacterium psychrophilum, the cause of coldwater or peduncle disease in salmonid and other cold freshwater species,6 and Tenacibaculum maritimum, in marine fish.1 Collectively, bacteria in this gram-negative genus average 2-5 μm in length, although forms up to 40 μm can occur. Longer rods are flexible and move by gliding motility. Colonies are typically yellow, a product of non-diffusible carotenoid or flexirubin-type pigment production.2. Isolation of the bacteria in culture requires the use of low nutrient agar media, such as Shieh or tryptone yeast extract salts (TYES). The organism will now grow on standard bacterial media such as trypticase soy agar (TSA). Confirmatory diagnostic tests include ELISA, FA, and LAMP methods, as well as conventional and qPCR procedures.3
Contributing Institution:
Department of Pathology, College of Veterinary Medicine, University of Georgia, Athens, GA 30602, www.vet.uga.edu/VPP
JPC Diagnosis: Head: Dermatitis,
necrotizing, and lymphocytic, focally extensive severe, with skeletal
muscle degeneration and necrosis and innumerable filamentous bacilli.
JPC Comment: The contributor has given us an outstanding review of this common bacterial pathogen of a wide range of freshwater fish. Flavobacterium columnare has been seen as a causative factor in a number of large-scale fish dieoffs, to include multiple species in separate reported dieoffs in the Buffalo Pound and Blackstrap Lakes, which affected yellow perch and lake whitefish respectively, and several thousand carp in the St. Lawrence river.8 In these mortality events, F. columnare was considered one of a number of factors, to include environmental stress due to ambient heat and lessened oxygen availability, and other bacterial pathogens, including Aeromonas hydrophila which was co-cultured from the dead fish.8
Increased water temperature has been noted as a major contributoring factor to the increase in F. columnare infections on a global basis. It was identified in a study by Pulkkinen et al. as a major factor in the increase in virulence of F. columnare over a period of 23 years in salmon farms in Finland. In addition to higher temperatures causing stress to fish,6 global warming also extends the periods in which F. columnare can grow and cause outbreaks, as well as facilitate faster tissue invasion by virulent strains as increase in chondrolysin lyase activity (facilitating dermal invasion) is seen at higher temperatures.3 In farmed fish, increased stocking levels contribute to outbreaks of F. columnare by increasing the organic load in the water, as well as nitrate concentration and the possibility for co-infections with other bacterial pathogens or ectoparasites.3
The moderator commented on the acute nature of the lesion which was illustrated by the lack of granulomatous inflammation, and believes that many of the inflammatory cells present within the lesion are lymphocytes and few are actually tissue macrophages (as would fit with the history of fish death within 24 hours after the stress of transport, as well as the normal progress of the disease - "the fish go down quickly".) as bacteria may be lost before autopsy, the moderator recommends wet-mounted skin scrapings in animals that are still living. After approximately 15 minutes, F. columnare will assume a typical "haystack" formation for which it is famous.
A differential diagnosis for facial necrosis in ornamental fish is spaC-type Erysipelothrix.5 This report describes a disease of ornamental fish resulting in facial cellulitis, necrotizing dematitis and myositis and disseminated coelomitis with numerous colonies of gram-positive organisms. Erysipelothrix was recovered by from numerous animals but were genetically divergent from existing species of E. rhusiopathae and E. tonsillarum known to be pathogenic in fish and marine mammals.5
References:
1 Avendano-Herrera R, Toranzo AE, Magariños B. Tenacibaculosis infection in marine fish caused by Tenacibaculum maritimum: a review. Dis Aquat Org. 2006; 71: 255-266.
2. Bernardet J âF, Segers P, M. Vancanneyt M, Berthe F, Kersters K, Vandamme P. Cutting a Gordian Knot: Emended classification and description of the genus Flavobacterium, emended description of the family Flavobacteriaceae, and proposal of Flavobacterium hydatis nom. nov. (Basonym, Cytophaga aquatilis Strohl and Tait 1978) Int J Syst Bacteriol. 1996; 46: 128-148.
3. Declerq AM, Haesebrouck F, Van den Broeck W, Bossier P, Decostere A. Columnaris disease in fish: a review with emphasis on bacterium-host interactions. Vet Res. 2013; 44: 27.
4. Durburow RM, Thune RL, Hawke JP, Camus AC. Columnaris disease, a bacterial infection caused by Flavobacterium columnare. Southern Regional Aquaculture Center Publication No. 479. 1998.
5. Pomaranski EK, Reichley SR, Yanong, R, Shelley J, Pouder DB, Wolf JC, Kenelty KV, Ven Bonn B, Oliaro F, Byrne B, Clothier KA, Griffen MJ, Camus AC, Soto E. Characterizatioj of spaC-type Erysipelothrix sp. isolates causing systemic disase in ornamental fish. J Fish Dis 2017; 41(1): 49-60.
6. Pulkkinen K, Suomalainen LR, Read AF, Ebert D, Rintamakk P, Valtonen ET. Intensive fish farming and the evoluation of pathogen virulence: the case of columnaris disease in Finland. Proc R Soc B 2010; 277:593-600
7. Starliper CE. Bacterial coldwater disease of fishes caused by Flavobacterium psychrophilum. J Adv Res. 2011; 2: 97-108.
8. Scott SJ, Bollinger TK. Flavobacterium columnare: an important contributing factor to fish die-offs in southern lakes of Saskatchewan, Canada. J Vet Diagn Investig 2014; 26(6)832-835.