Signalment:
Gross Description:
Histopathologic Description:
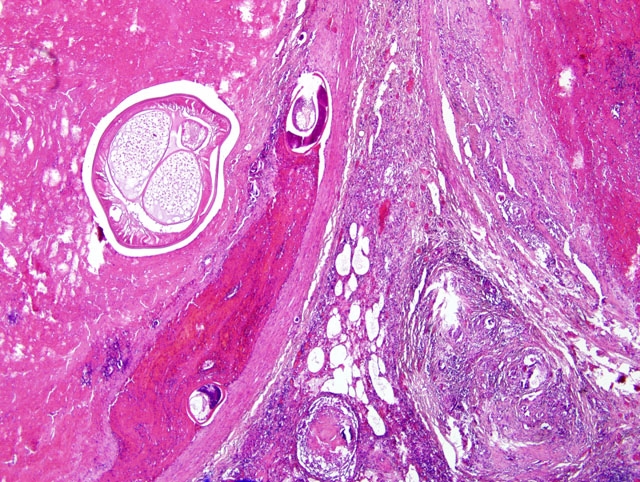
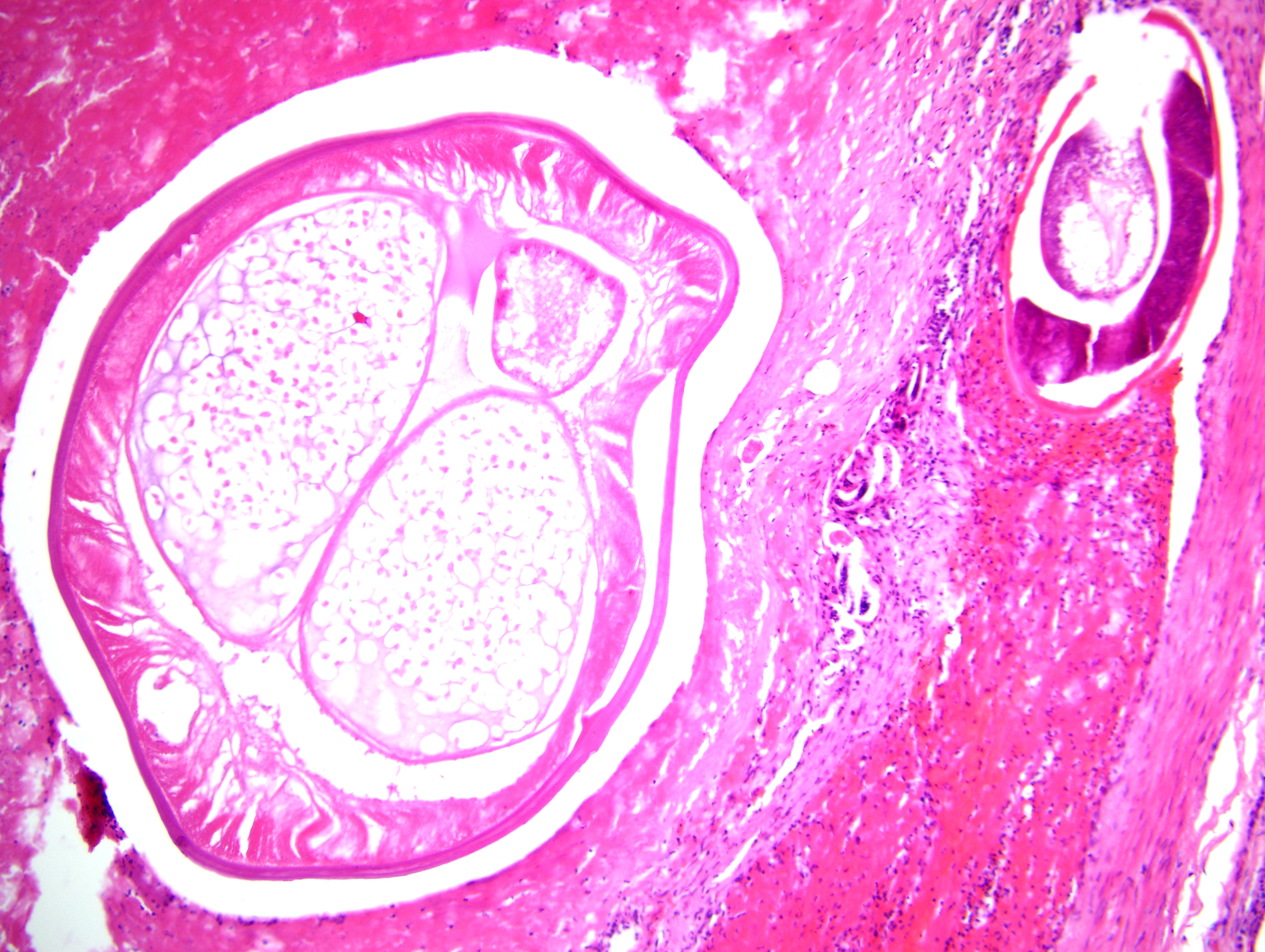
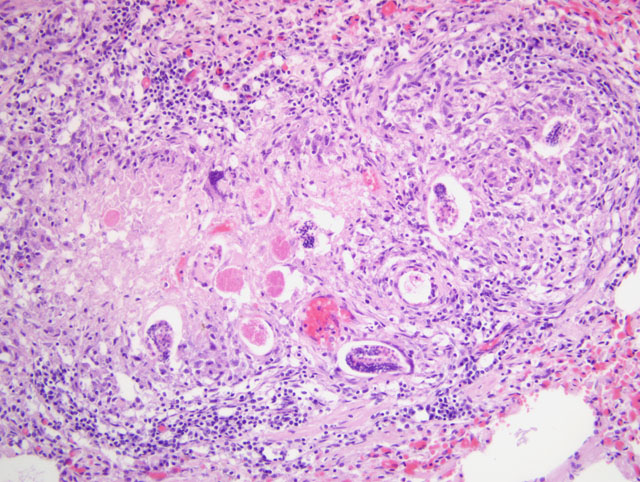
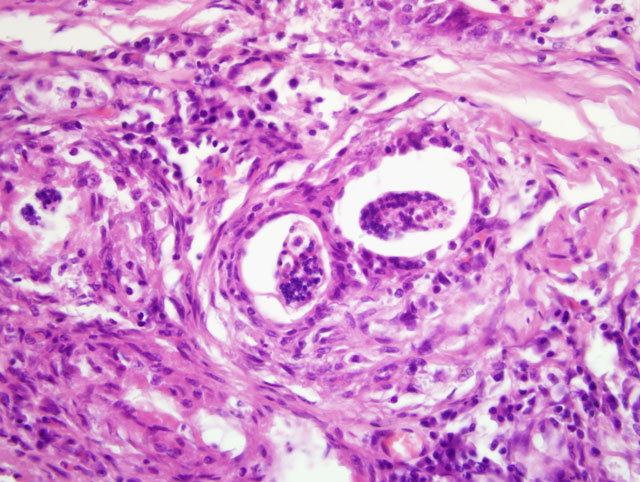
Focally, a pulmonary artery is severely dilated with the tunica intima thickened by moderate amount of fibrous connective tissue and few lymphocytes and plasma cells. Within the lumen there is a cross section of an adult, 0.5-1 mm in largest diameter (varies among sections and not present in all the sections), degenerate nematode with a thin eosinophilic cuticle, lateral cords, tall coelomyarian/polymyarian musculature, and a small intestine (consistent with a Dirofilaria immitis adult). Additionally in some sections, within this vessel and in an adjacent small artery, there are one to three tranverse sections of a smaller degenerate nematode (0.1-0.25 mm in largest diameter) characterized by a very thin coelomyarian musculature and a variably evident large strongyloid intestine; one nematode has a uterus. Intravascular nematodes are surrounded by thrombi, which focally adhere to the vessel walls. The thrombus is composed of fibrillar eosinophilic material (fibrin) that is partially organized in fibrous connective tissue containing multiple small blood-filled channels (organization and recanalization). Within the thrombi and in some vessel lumina there are occasional nematode larvae, consistent with Dirofilaria immitis microfilariae. The tunica media of numerous pulmonary arteries is markedly thickened (smooth muscle hypertrophy).
Morphologic Diagnosis:
1. Lung: severe, multifocal to coalescing, chronic, interstitial granulomatous pneumonia with intralesional nematode eggs and larvae.
2. Lung, pulmonary arteries: severe, focal, chronic, proliferative endarteritis with thrombus formation and an intralesional nematode adult and larvae.
Lab Results:
Condition:
Contributor Comment:
The first, second, and early third larval stages of D. immitis are obligate parasites of mosquitoes of the genera Aedes, Culex, and Anopheles. After maturation, larvae migrate into the cephalic spaces of the mosquito head and enter soft tissues of the new host when the mosquito feeds. The larvae reach the right ventricle 3-4 months after entering definitive host. Adults may live for years and microfilariae as long as 2.5 years. Clinical signs of canine dirofilariasis, such as cough and exercise intolerance, are related to cardiovascular dysfunction that may progress to congestive heart failure. The disease is seen more frequently in dogs older than 5 years, and clinical signs roughly correlate with the severity of infection. However, clinically normal dogs may harbor up to 30 worms. Adult heartworms are generally found in the pulmonary arteries and right ventricle; however, they may locate in vena cava or left ventricle in heavy infestations (i.e. 50 or more worms). The caudal lobar pulmonary arteries are generally most severely affected. Right heart failure may be caused by pulmonary hypertension due to vascular lesions.(7)
Immature and mature adult worms induce endarteritis with infiltration of eosinophils and neutrophils. Vascular lesions seem more severe in the presence of Wolbachia, a bacterial organism harbored by D. immitis.(5) The initial inflammation is followed by myointimal proliferation at sites of direct contact with worms; this reaction to chronic irritation is likely mediated by platelet-derived growth factor (PDGF). The lesions are followed by fibromuscular vascular wall hyperplasia. Pulmonary thromboembolism of viable or dead filarids (e.g. after therapy) may worsen hypertension, due to granulomatous interstitial inflammation elicited, and by occlusion of pulmonary vessels. However, pulmonary infarction seems an uncommon event.
In the pulmonary parenchyma, the most common microscopic findings associated with D. immitis infection are arterial thrombosis and periarterial granulomatous inflammation.(7) Additional complications of heartworm disease include progressive chronic right heart failure with chronic passive congestion of the liver and occasional ascites. The vena caval syndrome is usually seen in young dogs with large numbers of adult worms that fill the right atrium and the vena cava, most likely as a result of retrograde migration from pulmonary arteries. In these instances, dogs develop sudden weakness, anorexia, bilirubinuria, hemoglobinuria and anemia. Shock derives from venous obstruction and decreased venous return. A common renal lesion associated with D. immitis infection is a membranoproliferative glomerulonephritis derived from the deposition of immune complexes from the circulation or formed in situ. The immune response leading to immune complex formation may be elicited by adults, immature adults, and microfilariae.(10) The most frequent ultrastructural lesions observed in kidneys of affected dogs are thickening of the glomerular basement membrane zone, presence of dense deposits in the glomerular basement membrane, and effacement of foot processes. In some dogs, electron dense deposits may be seen in the mesangium with expansion of mesangial matrix.(10)
Cats may become infected with D. immitis and are typically microfilaremic or afilaremic due to the low number of adults (often one) and high frequency of filarial male-only infection. Cats may develop cough and dyspnea, vomiting, and neurological signs, or sudden death may be the only event. A significant decrease in pulmonary intravascular macrophage activity in cats with D. immitis infection has been identified.(3)
Dirofilaria sp. may occasionally infect man.(9,11,13) The infection in America is more commonly attributed to D. immitis, while in Europe, pulmonary dirofilariasis is most commonly attributed to aberrant migration of D. repens. (11) There is no clear explanation for this geographical selective distribution. The distribution of reported human cases seems to reflect the prevalence of the disease in the canine population in different areas of the United States (13). These filarids induce a focal to multifocal pulmonary granulomatous reaction termed coin lesions.(9,13) Coin lesions are the end-stage tissue result of the parasites death in the vascular bed and the stimulation of a granulomatous reaction. Although the disease still seems rare in humans, serology should be performed in regions where D. immitis is highly enzootic. D. immitis coin lesions present significant differential diagnostic problems since they may resemble the lesions of tuberculosis, fungal infections, or neoplasia.(13)
Angiostrongylus vasorum can be differentiated from D. immitis by histopathology, as described above, or by examination of intact adults. Intact adult A. vasorum females have a barber-pole appearance due to the helicallyarranged red digestive tract and white ovaries. A. vasorum has a thin coelomyarian musculature, a large strongyloid intestine composed of few multinucleated cells, and a uterus with eggs.(2,4)
A. vasorum, termed French heartworm because it was reported first in France in the 1800s, is a metastrongylid nematode considered to be the most pathogenic lungworm of dogs.(1,12) Adults reside in the pulmonary artery and right heart ventricle in canids. Red foxes are the natural definitive hosts and are important reservoirs of infection for domestic dogs. Clinical signs may vary from mild coughing and exercise intolerance to fatal cardiopulmonary disease. A. vasorum has a worldwide distribution and the infection seems to be increasing in recent decades.(12) A. vasorum has an indirect life cycle in which aquatic and terrestrial snails serve as intermediate hosts.(1) Adult female nematodes shed eggs that are transported to the pulmonary parenchyma. Eggs develop and hatch, releasing first stage larvae that penetrate the alveoli. Larvae are coughed, swallowed, and excreted in the feces. Gastropods become infected with L1 larvae by eating contaminated plant material. In gastropods, L1 mature into L3 larvae. Final hosts become infected by ingestion of snails. Third stage larvae penetrate the gastrointestinal tract, migrate through visceral lymph nodes, and develop into immature adults. Juvenile worms migrate into the caudal vena cava trough the portal circulation and reach maturity in the pulmonary arteries.
A. vasorum may cause right heart failure and extensive pulmonary lesions as a consequence of egg embolization. Gross lesions consist of small, 1-2 mm diameter, red, firm, multinodular to confluent areas of hemorrhage and edema at the lung periphery. A variably severe, multifocal to coalescing, granulomatous to pyogranulomatous interstitial pneumonia with presence of eggs and larvae has been reported, often associated with prominent pulmonary arterial thrombosis. Vascular lesions are characterized by proliferative endarteritis, including thrombosis, thickening of tunica intima by fibromuscular tissue, and medial hypertrophy with infiltration of eosinophils, lymphocytes and plasma cells. Fibrosis and vascular recanalization of arterial thrombi may be seen in chronic cases. Granulomas have also been reported to occur in lymph nodes, brain, kidneys, and adrenal glands.(1,2)
The genus Angiostrongylus includes other important parasites, such as A. cantonensis, the rat lungworm. This parasite has an obligate neural migration cycle that may cause ascending paralysis and lumbar hyperalgesia in accidental hosts, such as dogs.
The differential diagnosis for pulmonary parasitic infections in dogs includes Crenosoma vulpis, Eucoleus aerophilus (formerly Capillaria aerophila), Oslerus osleri (formerly Filaroides osleri), Filaroides hirti and Andersonstrongylus milksi (formerly Filaroides milksi).(2) C. vulpis infects bronchioli, bronchi and the trachea of wild canids and occasionally domestic canids, leading to chronic coughing.(2,12)
Eucoleus aerophilus is a trichurid nematode that parasitizes wild carnivores, domestic cats, and rarely dogs. The adult lungworms live embedded in the respiratory lining epithelium. Most infections are inapparent; more severe infection may result in catharral inflammation, coughing, secondary bacterial infection, and/or airway obstruction. The adult nematodes are slender, 2-3 cm long, and characterized by presence of bacillary bands (segmental thickenings of the hypodermis), a stichosome (basophilic esophageal gland), and embryonated eggs in the uterus. Eggs are oval with bipolar plugs; their morphology allows differentiation from other nematodes. Females lay eggs where larvae undergo initial development. Eggs are swallowed and are released via the feces. Animals become infected by ingesting embryonated eggs or earthworms bearing embryonated eggs. Hatched larvae migrate to the lungs, where they become adults in 3 to 6 weeks.(2,12)
Oslerus osleri is commonly reported in wild canids.(2) The infection is generally asymptomatic. The parasite induces the formation of single to multiple, 1-10 mm diameter, firm, submucosal nodules in the trachea and bronchi that are most prominent at the tracheal bifurcation. Coiled worms may be grossly visible trough the overlying mucosa. Dead worms may cause a granulomatous to pyogranulomatous reaction. Nodules are circumscribed by fibrous tissue and contain adult parasites or fifth stage larvae. Diagnosis is based on adult worm identification by histology or from crush preparations of tracheal nodules. The adults have coelomyariam musculature, an intestine composed of few multinucleated cells with indistinct microvilli, and embryonated eggs or larvae in the uterus.
Filaroides hirti has been reported in colonies of laboratory beagles with cases described in pet dogs.(2) F. hirti has a direct life cycle wherein infective first stage larvae are passed in the feces. Most infections are apparently acquired from the dam. Adults live in the alveoli and respiratory bronchioles. Mostly incidental findings at necropsy, lesions are 1-5 mm diameter gray-tan to black-green nodules in subpleural regions of lungs. Nodules may be white or have cystic centers. Rarely, fatal cases occur in immunosuppressed dogs; in these cases, a severe granulomatous pneumonia has been reported. Histopathology consists of minimal inflammation in response to live adult worms; however, dead parasites induce severe interstitial granulomatous and eosinophilic inflammation.
Andersonstrongylus milksi is a metastrongylid nematode. The literature regarding this parasite is confusing (2) and identification of the adult is necessary to distinguish A. milksi from F. hirti. Adults inhabit bronchioles and alveoli and the gross and histologic lesions are similar to those caused by F. hirti. Larvae have been reported in the brain and internal abdominal organs.
JPC Diagnosis:
1. Lung, arteries: Endarteritis, proliferative, chronic, multifocal, marked, with organizing thrombi and few intravascular adult metastrongylid and filarid nematodes, etiology consistent with Angiostrongylus vasorum and Dirofilaria immitis.
2. Lung: Pneumonia, granulomatous, multifocal coalescing, marked, with hemorrhage, fibrosis, and many nematode larvae and eggs.
Conference Comment:
Conference participants reviewed the classic immune response to invasive nematodes, in which a TH2 response generally predominates. Briefly, cytokines produced by cells of the innate immune system in response to microbes activate and direct the differentiation of helper T cells in the adaptive immune system. In response to invasive nematodes, interleukin (IL)-4 initiates the differentiation of na+�-�ve T cells into TH2 cells. This differentiation requires the lineage-specific transcription factors GATA3 and c-Maf; differentiated TH2 cells characteristically produce IL-4, IL-5, and IL-13. Interleukin-4 acts on B cells to stimulate class-switching to IgE, which mediates the cross linking of Fc receptors on mast cells and their activation. Additionally, IL-4 further amplifies the differentiation of TH2 cells in an autocrine loop, and inhibits the differentiation of TH17 cells, potent recruiters of neutrophils and monocytes involved in the host defense against extracellular bacteria and fungi. Interleukin-5 activates eosinophils, and IL-13 enhances IgE production, stimulates mucus production by epithelial cells, and stimulates the synthesis of proline, an important constituent of collagen, thereby stimulating fibrosis.(6,8) Conference participants noted the paucity of eosinophils present in this case and speculated on possible explanations, such as glucocorticoid administration or immunosuppression.
References:
2. Caswell JL, Williams KJ: Respiratory system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 3, pp. 645-648. Saunders Elsevier, Philadelphia, PA, 2007
3. Dillon AR, Warner AE, Brawner W, Hudson J, Tillson M: Activity of pulmonary intravascular macrophages in cats and dogs with and without adult Dirofilaria immitis. Vet Parasitol 158:171-176, 2008
4. Gardiner CH, Poynton SL: An Atlas of Metazoan Parasites in Animal Tissues. Armed Forces Institute of Pathology, Washington, DC, 1999
5. Kramer L, Grandi G, Leoni M, Passeri B, McCall J, Genchi C, Mortarino M, Bazzocchi C: Wolbachia and its influence on the pathology and immunology of Dirofilaria immitis infection. Vet Parasitol 158:191-195, 2008
6. Kumar V, Abbas AK, Fausto N, Aster JC: Diseases of the immune system. In: Robbins and Cotran Pathologic Basis of Disease, eds. Kumar V, Abbas AK, Fausto N, Aster JC, 8th ed., pp. 183-201. Saunders Elsevier, Philadelphia, PA, 2010
7. Maxie MG, Robinson WF: Cardiovascular system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 3, pp. 88-89. Saunders Elsevier, Philadelphia, PA, 2007
8. Miossec P, Korn T, Kuchroo VK: Interleukin-17 and type 17 helper T cells. N Engl J Med 361:888-898, 2009
9. Narine K, Brennan B, Gilfillan I, HIdge A: Pulmonary presentation of Dirofilaria immitis (canine heartworm) in man. Eur J Cardiothor Surg 16:475-477, 1999
10. Paes-de-Almeida EC, Ferreira AMR, Labarthe NV, Cladas MLR, McCall JW: Kidney ultrastructural lesions in dogs experimentally infected with Dirofilaria immitis (Leidy, 1856). Vet Parasitol 113:157-168, 2003
11. Pampiglione S, Rivasi F, Gustinelli A: Dirofilarial human cases in the Old World, attributed to Dirofilaria immitis: a critical analysis. Histopathology 54:192-204, 2009
12. Taubert A, Pantchev N, Globokar Vrhovec M, Bauer C, Hermosilla C: Lungworm infections (Angiostrongylus vasorum, Crenosoma vulpis, Aelurostrogylus abstrusus) in dogs and cats in Germany and Denmark in 2003-2007. Vet Parasitol 159:175-180, 2009
13. Theis JH: Public health aspects of dirofilariasis in the United States. Vet Parasitol 133:157-180, 2005
14. Traversa D, Di Cesare A, Milillo P, Iorio R, Otranto D. Infection by Eucoleus aerophilus in dogs and cats: is another extra-intestinal parasitic nematode of pets emerging in Italy? Res Vet Sci 87:270-272, 2009