Signalment:
A 2-year-old, intact female Maltese dog was presented to the veterinarian with a history of acute neurological signs characterized by seizures, constant howling, and circling to the right side. The owner informed that the immunization schedule had been followed. On neurological examination, the dog presented deficit of mental status (apathy and depression), head turn, compulsive circling to the right side and falls to the left side. During ambulation the animal turned away from obstacles, indicating no visual deficit. Abnormal movements and proprioception deficit to the left side were detected. There was also left deficit to menace response and cervical sensibility. A multifocal intracranial lesion involving the telencephalon was suspected. The complete blood count and serum chemistry profiling were unremarkable. Magnetic resonance imaging (MRI) and CSF analysis could not be performed because of the clients refusal. According with breed, age, history and neuroanatomic localization of the lesions, an inflammatory neurological disease of unknown cause was suspected. On the basis of the suspicion, phenobarbital (6mg/kg bid), doxycycline (10mg/kg bid), prednisolone (2mg/kg bid), and A and B vitamins were prescribed. A re-check five days after the onset of therapy was performed and no changes were detected when thorough clinical examination was performed. On neurological examination, reduction of circling and seizures was observed but there was no improvement in posture, proprioception and menace response. Because clinical signs improved gradually, the initial prescription was maintained. Fifty three days after the first presentation the dog was checked again. The owner reported that the initial dosage of prednisolone was maintained but, arbitrarily the client did not maintained the medicine after three weeks, and the seizures episodes started again after discontinuing the corticoid therapy. The veterinarian detected that all clinical signs mentioned above worsened. A new treatment protocol adding cyclosporine (6 mg/kg bid) to prednisolone was prescribed. However, ten days later, the clinical signs worsened dramatically. The dog presented severe changes of the mental status (disorientation, aggression and apathy), increased postural deficit, and bilateral deficit to menace response and cluster seizures. Due to the poor prognosis, the owner elected euthanasia.
Gross Description:
Histopathologic Description:
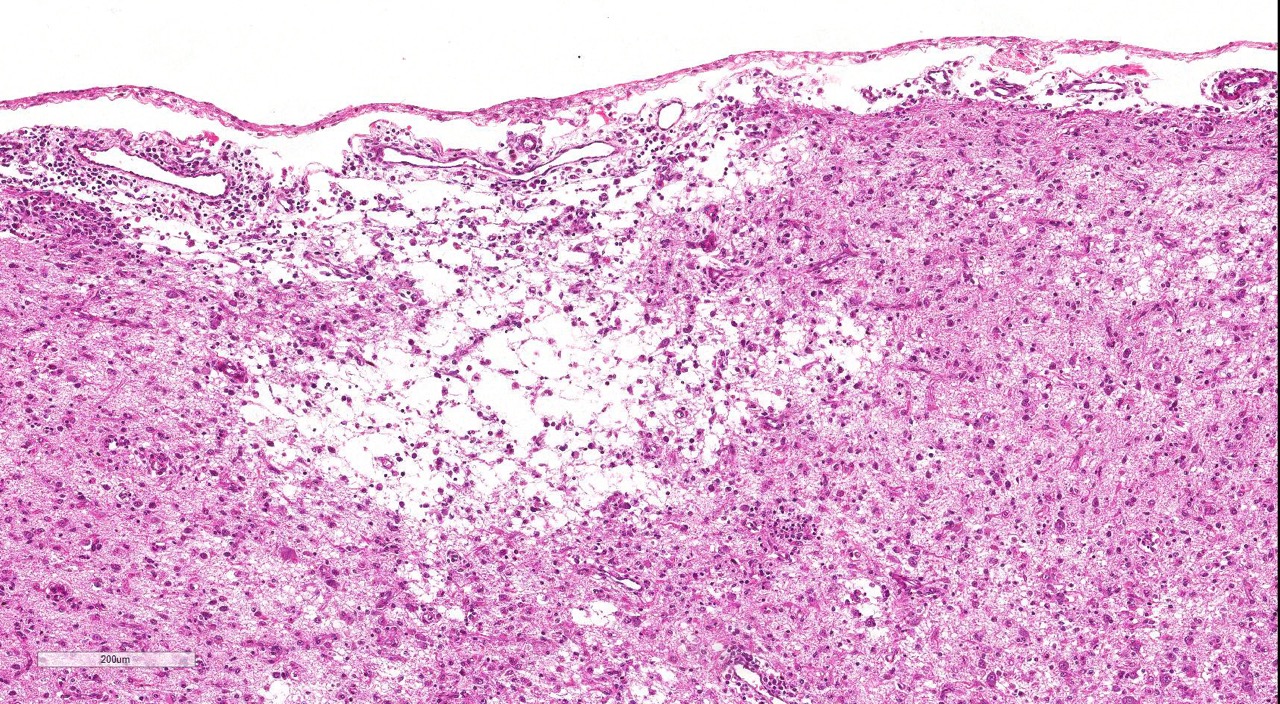
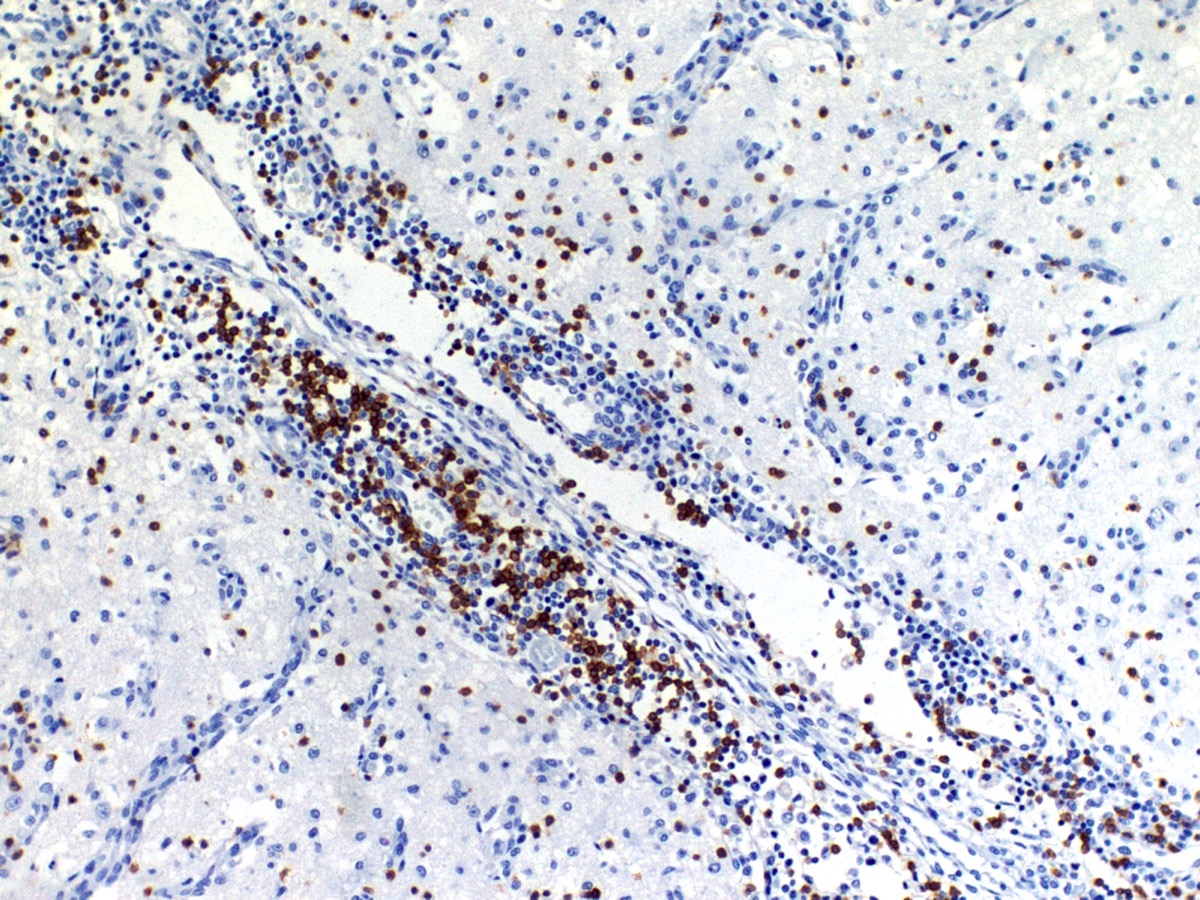
The cerebral cortex showed areas with markedly increased cellularity interspersed with multifocal cavitation, partially filled by numerous Gitter cells, characterizing malacia. Moderate to marked infiltration of cells, such as plasma cells and lymphocytes were observed around vessels and diffuse in the leptomeninges. In addition, the non-cavitation areas were characterized for neuropil vacuolization, neuronal necrosis, neuronophagia, astroglyosis with various gemistocytes, endothelial hyperplasia and hypertrophy. Single or binucleate gem-istocytes were more commonly seen adjacent to necrotic areas. These areas were more intense in the frontal and parietal lobes of right side. In the white matter subjacent to the necrotic right frontal cortex there were plasma cells and lymphocytes perivascular cuffs associated to mild vacuolization and axonal degeneration. No lesions were found in the hippocampus, diencephalon (thalamus and hypothalamus), mesencephalon, cerebellum and medulla oblongata. Paraffin blocks of the cerebrum were selected and immunohistochemistry for CD3 (lym-phocytes T marker) and CD 79a (lymphocytes B marker) was performed. The immunohistochemical analysis showed that positive CD3 cells were predominant in the perivascular cuffs, leptomeninges and also in the neuroparenchyma. Less numbers of positive CD79a cells were observed in the perivascular cuffs and leptomeninges but they were rarely observed in the neu-roparenchyma.
Morphologic Diagnosis:
Cerebrum: marked multifocal to coalescing, necrotizing non-suppurative meningoencephalitis.
Lab Results:
Condition:
Contributor Comment:
NME has been reported in various toy breeds including Pug dogs, Yorkshire terrier, Maltese, Chihuahua, Shih Tzu, West highland white terrier, Boston terrier, Spitz Japanese and Pinscher, Pekingese and French bulldog.9,13 NME seems to be more common in females5,8 and, has been diagnosed in dogs with six months to seven years of age.13 However, the most common age range is from two13 to four years.5
The clinical signs associated with NME are rapidly progressive and associate to the neuroanatomical localization.13 The most common signs include seizures, depression, circling, visual deficit,5,6 postural reaction deficits4 and vestibule-cerebellar signs.6,12 Definitive antemortem diagnosis is challenging because histopathology is mandatory. For most cases, the clinical diagnosis is presumptive, associating clinical signs and neuroanatomic lo-calization, CSF analysis and advanced imaging tests to exclude other causes.5,12,13 The prognosis of NME cases is poor due the progressive course of the disease, with lower survival rate in animals with seizures.5 The dog of the present study showed clinical signs rapidly progressive and associated with the neuroanatomic localization of the lesions as observed in other studies.13 The lesions and clinical signs of most cases of NME are related exclusively to the cerebrum, being an important characteristic for this condition.12 Detailed neurological examination allows to determining the sites of the lesions, which are extremely important for presumptive diagnosis and treatment. In addition to neurological examination for supporting de clinical su-spicion, is fundamental the epidemiological data, CSF analysis, cross-sectional imaging via computed tomography (CT) scan or magnetic resonance imaging (MRI) of the CNS and infectious diseases testing. Ct-guided brain biopsy and histopathological evaluation of brain tissue may be considered in cases of suspected NME.5,13
An autoimmune pathogenesis has been suggested for NME based on the presence of anti-astrocytic and anti-glial fibrillary acid protein (GFAP) autoantibodies in the cerebrospinal fluid (CSF) of affected dogs.14 However, similar antibody levels occur in the CSF of dogs with GME, brain tumors and even in some clinically normal dogs.14 A genomic study in Pugs with NME showed a single strong association with dog leukocyte antigen (DLA) class II, and supports the role of the immune system in the disorder.6 Genetic predisposition also has been confirmed in Pug dogs with NME but it is believed there are additional influences contributing to the phenotypic expression of the disease.2,6
The immunohistochemical study showed predominance of T lymphocytes in the leptomeninges, around vessels and in the neuroparenchyma similar to the observed in other studies in dogs with NME.7,12 A study using double-labeling immunofluorescence antibody demonstrated predominance of CD3+ T lymphocytes in close proximity or attached to astrocytes, and the cytoplasm and astrocytic processes were positive for IgG in the NME and NLE lesions. The involvement of the autoantibody to astrocytes in the NME cases supports the immune mediated pathogenesis hypothesis however, does not confirm if anti-astrocytic and anti-GFAP antibodies are a primary cause or a secondary consequence of NME.12 A recent study showed that viral pathogens are not common in the brain of dogs with NME.1
JPC Diagnosis:
Conference Comment:
Although most commonly associated with small/toy breed dogs, NME has also been reported in a Staffordshire bull terrier.4 Other small breeds not mentioned above that have been documented with NME include Coton de Tulear, Papillon, and Brussels Griffon although it is very uncommon in these breeds and their development of this condition is considered atypical.3 The pathogenesis involved in development of this condition in atypical breeds is unclear as much remains elusive regarding the development, progression and pathogenesis of NME, as well as NLE and GME. In this case, the lesions were fairly characteristic for NME, particularly given the signalment.However, in some early or mild cases in less commonly affected breeds, there may be clinical and/or pathologic overlap with infectious diseases such as Neospora caninum, Toxoplasma gondii and viruses (e.g., canine distemper virus). Therefore, when the diagnosis is less straightforward but NME is still considered a top differential diagnosis, it is important for the diagnostic pathologist to consider and rule out infectious causes.
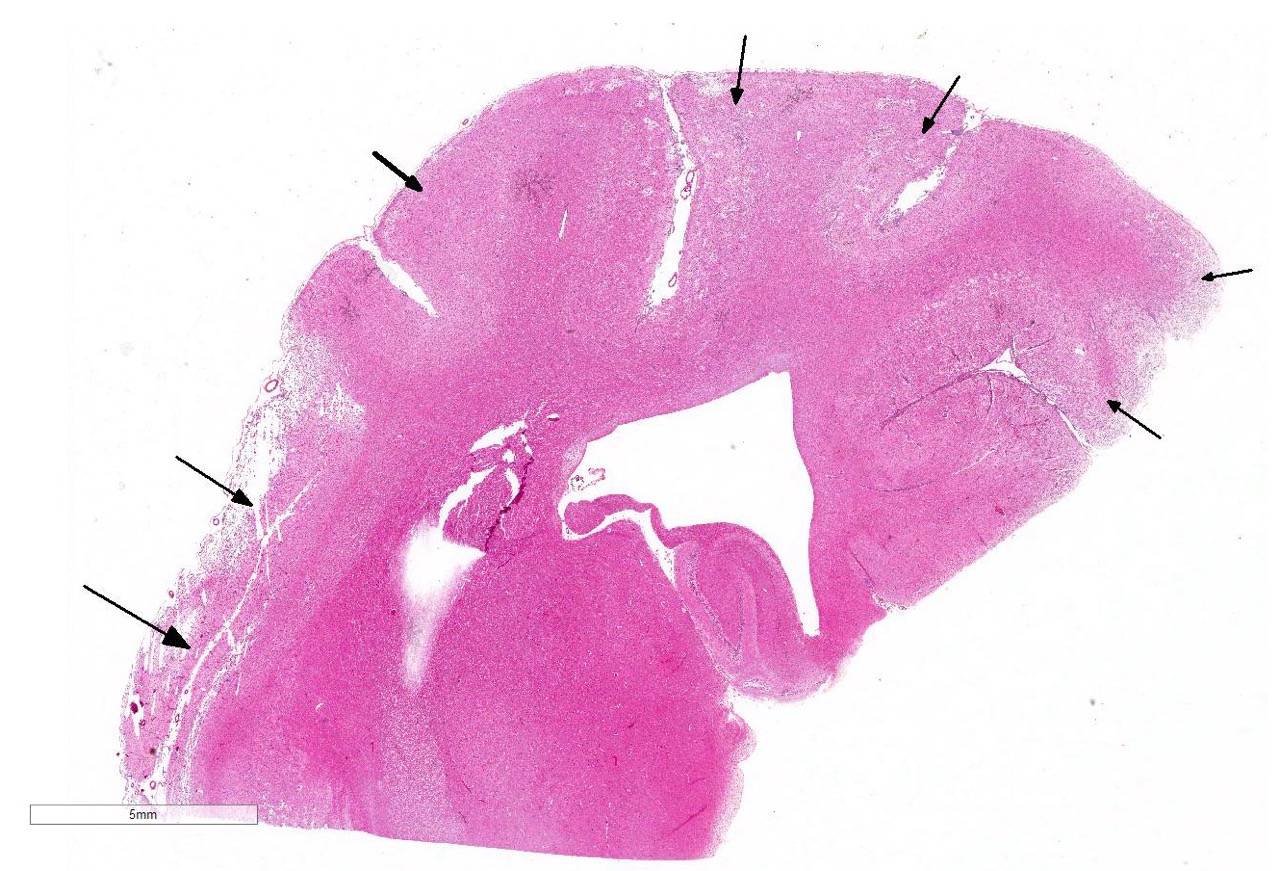
The subgross view of this section is striking and the lesions are
characteristic for this entity. Within up to 50% of the section, there is loss
of differential staining with areas of pallor, drop-out and liquefactive
necrosis localized to the grey matter.Within necrotic
areas, there is loss of neuropil and increased numbers of microglial cells, lymphocytes,
and plasma cells; moderate numbers of gitter cells and hypertrophied astrocytes
are also present.There is multifocal perivascular cuffing in the grey matter
with affected vessels have reactive endothelium.Spongiosis is present multifocally and necrotic
neurons are seen in low numbers.The meninges are mul-tifocally expanded with
edema and infiltrated by macrophages, lymphocytes and plasma cells.Conference
participants discussed and contrasted the histologic lesion described here to
what was seen in the GME case. Ventricular dilation and hy-drocephalus ex-vacuo
were also described; however, the moderator cautioned against over-interpreting
this change in bra-chycephalic breeds which may normally have some degree of
ventricular dilation. 1.
Barber RM, Porter BF, Li Q, et al. Broadly reactive polymerase
chain reaction for pathogen detection in canine granulomatous
meningoencephalomyelitis and necrotizing meningoencephalitis. J Vet Intern
Med. 2012; 26(4):962-968. 2.
Barber RM, Schatzberg SJ, Corneveaux JJ, et al. Identification of
risk loci for necrotizing meningoencephalitis in pug dogs. J. Hered.
2011;102: 540-546. 3.
Cooper JJ, Schatzberg SJ, Vernau KM, Summers BA, et al.
Necrotizing Meningoencephalitis in atypical dog breeds: A case series and
literature review. J Vet Intern Med. 2014; 28:198-203. 4.
Estey CM, Scott SJ, Cerda-Gonzalez S. Necrotizing
Meningoencephalitis in a large mixed-breed dog. J Am Vet Med Assoc. 2014;
245(11):1274-8. 5.
Granger N, Smith PM, Jeffery ND. Clinical findings and treatment
of non-infectious meningoencephalomyelitis in dogs: A systematic review of 457
published cases from 1962 to 2008. Vet J. 2010; 184: 290-297. 6.
Greer KA, Schatzberg SJ, Porter BF, et al. Heritability and
transmission analysis of necrotizing meningoencephalitis in the Pug. Res.
Vet. Sci., 2009; 86: 438442. 7.
Higgins RJ, Dickinson PJ, Kube SA, et al. Necrotizing
meningoencephalitis in five chihuahua dogs. Vet Pathol. 2008; 45:
336346. 8.
Levine JM, Fosgate GT, Porter B, et al. Epidemiology of
Necrotizing Men-ingoencephalitis in Pug Dogs. J Vet Intern Med. 2008;
22: 961968. 9.
Park ES, Uchida K, Nakayama H. Comprehensive immunohistochemical
st-udies on canine necrotizing me-ningoencephalitis (NME), necrotizing
leukoencephalitis (NLE), and granulomatous meningoencephalomyelitis (GME). Vet
Pathol. 2012, 49 (4):682-92. 10. Schrauwen
I, Barber RM, Schatzberg SJ, Siniard AL, et al. Identification Identification of
Novel Genetic Risk Loci in Maltese Dogs with Necrotizing Me-ningoencephalitis
and Evidence of a Shared Genetic Risk across Toy Dog Breeds. PLoS One.
2014;9(11):e112755. 11. Shibuya
MN, Fujiwara K, Imajoh-Ohmi S, et al. Autoantibodies against glial fibrillary
acidic protein (GFAP) in cerebrospinal fluids from Pug dogs with necrotizing
meningoencephalitis. J Vet Med Sci. 2007; 69(3): 241-245. 12. Spitzbarth
I, Schenk HC, Tipold A, Beineke A. Immunohistochemical Ch-aracterization of
Inflammatory and Glial Responses in a Case of Necrotizing Leucoencephalitis in
a French Bulldog. J Comp Path. 2010; 142: 235-241. 13. Talarico
LR., Schatzberg SJ. Idiopathic granulomatous and necrotizing inf-lammatory
disorders of the canine central nervous system: a review and future
perspectives. J Sm Anim Pract. 2010; 51: 138-149. 14.Toda Y, Matsuki N, Shibuya M, et al. Glial fibrillary acidic
protein (GFAP) and anti-GFAP autoantibody in canine necrotising
meningoencephalitis. Vet Rec. 2007; 161(8): 261-264.
References: