Signalment:
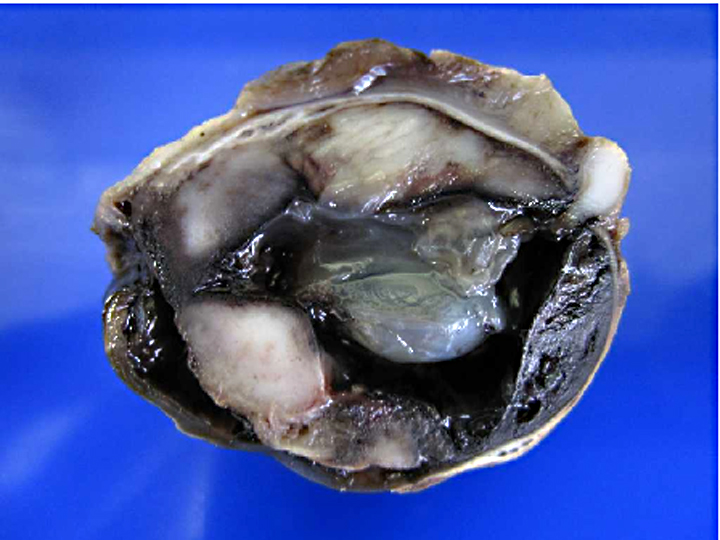
Gross Description:
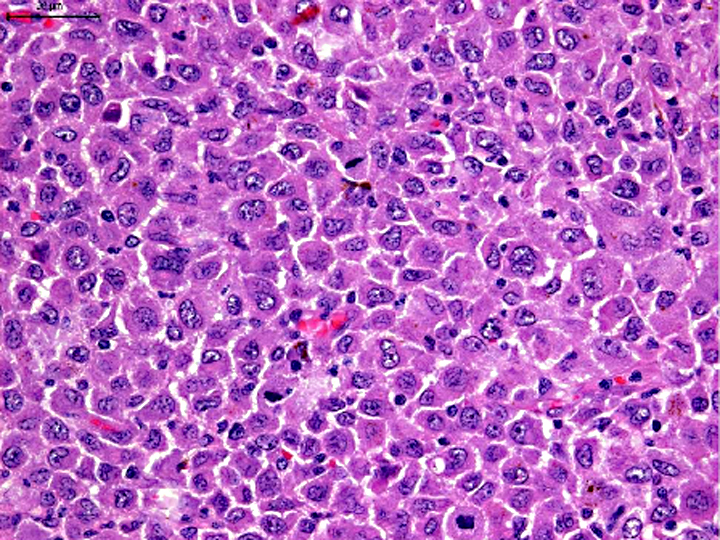
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Histiocytic sarcoma, which is identified as a single lesion of histiocytic neoplasia, is due to proliferation of dendritic antigen presenting cells (DC). The majority occurs in the subcutis, but other primary locations have been observed. Disseminated histiocytic sarcoma, which spreads to distant sites beyond the local lymph nodes, is an aggressive multisystem disease characterized by presence of multiple tumor masses in several organ systems. Primary sites are spleen, lung, and bone marrow. Secondly, lesions are observed in lymph nodes and liver, and subsequently other organs can be affected. When histiocytic neoplasia occurs in multiple sites simultaneously, the disease is termed malignant histiocytosis.(1)
Histiocytic sarcoma is an uncommon primary intraocular neoplasm, whereas life expectancy following diagnosis is very short compared to other ocular tumors. It is important that malignant melanoma, irido-ciliary epithelial tumor, and malignant lymphoma were eliminated from the differential diagnosis by histomorphologic examination. Melanocytic tumors arising from the uvea are the most commonly detected ocular tumors in dogs, and approximately 20% of these tumors are malignant melanoma. Irido-ciliary epithelial tumor is the second-most-common primary uveal tumor in dogs, accounting 12.5% of canine ocular tumors. Primary malignant lymphoma is the third-most-common intraocular tumor in dogs, representing 3.3% of canine ocular tumors.(9)
Primary intraocular histiocytic sarcoma has a strong breed association. Approximately 70% of these tumors occur in Retriever breeds accounting for Golden Retrievers and Labrador Retrievers.(9) Among other breeds Rottweilers are the third-most-common breed. On the other hand, histiocytic sarcoma and malignant histiocytosis is best recognized in the Bernese mountain dog, in which a familial association is apparent.(5) Other breeds are predisposed to histiocytic sarcoma and include Rottweilers, Golden Retrievers, and Flat-coated Retrievers. Because histiocytic sarcoma is characterized by rapid dissemination, it should be listed in differential diagnoses of intraocular tumors of Retriever breeds, especially Golden Retrievers and Labrador Retrievers.
Histiocytes differentiate from CD34+ myeloid stem cells into monocyte/macrophages and several dendritic antigen presenting cells (DC) lineages, which include epithelial DC or Langerhans cells, interstitial DC, plasmacytoid DC, and interdigitating DC of the paracortical area of the lymph node. Histiocytic sarcomas express leukocyte surface molecules characteristic of DC such as CD1, CD11c and MHC II.(1) Diffuse expression of E-cadherin, CD4 and Thy-1 has not been observed in histiocytic sarcoma. On the other hand, the phenotype of histiocytoma, which is a benign tumor of non-activated Langerhans cells, is quite similar to that of histiocytic sarcoma except for the expression of E-cadherin, which occurs in histiocytoma especially in the cellular infiltrate immediately adjacent to the epidermis. Reactive histiocytosis, which is a proliferative disease of activated interstitial DC, consistently expresses CD4 and Thy-1.(1)
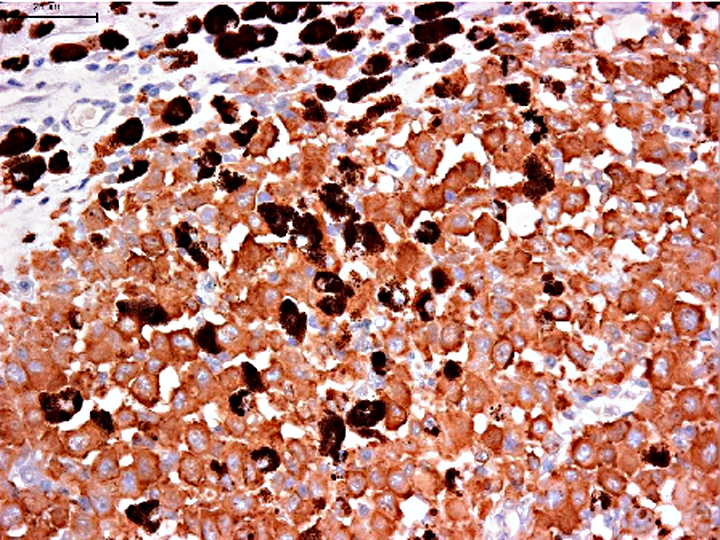
CD1, CD11c and CD4 antibodies could not be used on formalin fixed paraffin embedded specimens. So, we need to use the antibody panel for CD3 (T cells), CD20 (B cells), CD79cy (B cells), CD18 (macrophage, lymphocyte, granulocyte), MHC-II, Iba-1 (macrophage, microglia). MHC-II, Iba-1 and lysozyme expression with the absence of CD3, CD20 and CD79cy is evidence of macrophage and DC differentiation. Macrophages, which are specialized scavengers, have more numerous lysosomes than dendritic antigen presenting cell (DC). In the present case, the neoplastic cells have small numbers of lysosomes under electron microscopic examination. Thus, these neoplastic cells are regarded as DC rather than macrophages. In addition, neoplastic cells are negative for E-cadherin, which intra-epithelial DC such as Langerhans cells express. The exact sublineages of histiocytes and dendritic cells involved in HS have not been determined in most instances, but our present case is highly suggestive of interstitial DC origin.
JPC Diagnosis:
Conference Comment:
| Cell of origin | Disease(s) | Immunohistochemistry | |
| Positive | Negative | ||
| Epidermal dendritic (Langerhans) cell | Cutaneous histiocytoma; Feline PCLH (Birbecks granules on EM) | E-cadherin, CD1a, CD14, CD11c, CD18, MHC II, ICAM-1, langerin | Thy-1, CD4 |
| Interstitial dendritic cell | Reactive histiocytosis | Thy-1, CD4, CD1c, CD11b/c, MHC II, CD18 | ICAM-1, E-cadherin |
| Myeloid dendritic cell | Histiocytic sarcoma | ICAM-1, CD1, CD11c, MHC II, -� CD90 | CD4, E-cadherin |
| Macrophage | Hemophagocytic histiocytic sarcoma | CD11d (β2- integrin), MHC II, -� CD11c/CD18, CD1c | CD1c/CD11c |
References:
2. Affolter VK, Moore PF. Canine cutaneous and systemic histiocytosis. Am J Dermatopathol. 2000;22:40-48.
3. Baines SJ, McInnes EF, McConnell I. E-cadherin expression in canine cutaneous histiocytomas. Vet Rec. 2008;162(16):509-513.Â
4. Coomer AR, Liptak JM. Canine histiocytic diseases. Compend Contin Educ Vet. 2008;30(4):202-204.
5. Dubielzig RR, Ketring KL, McLellan GJ, et al. Uveal neoplasia. Veterinary ocular pathology. Philadelphia, PA: Saunders; 2010:282-309.
6. Fernandez NJ, West KH, Jackson ML, et al. Immunohistochemical and histochemical stains for differentiating canine cutaneous round cell tumors. Vet Pathol. 2005;42(4):437-445.
7. Ginn PE, Mansell JEKL, Rakich PM. Skin and appendages. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals 5th ed. vol. 1. Philadelphia, PA: Saunders Elsevier; 2007:768-769.
8. Goldschmidt MH, Hendrick MJ. Tumors of the skin and soft tissues. In: Meuten DJ, ed. Tumors in Domestic Animals. 4th ed. Ames, IA: Iowa State Press; 2002:109-111.
9. Moore PF, Rosin A. Malignant histiocytosis of Bernese mountain dogs. Vet Pathol. 1986:23:1-10.
10. Naranjo C, Dubielzig RR, Friedrichs KR. Canine ocular histiocytic sarcoma. Vet Ophthalmol. 2007 May-Jun;10(3):179-85.