Signalment:
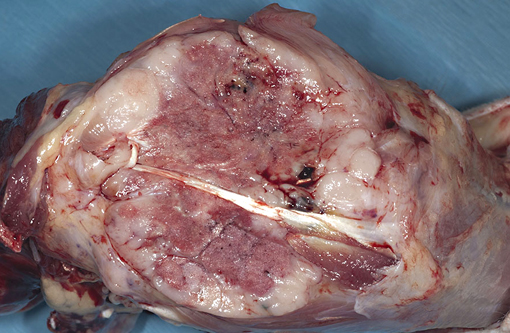
Gross Description:
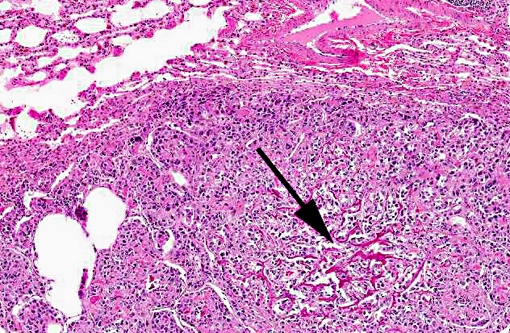
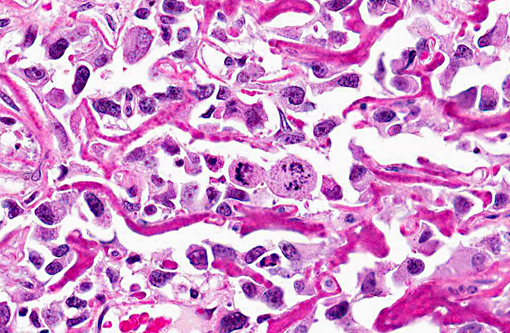
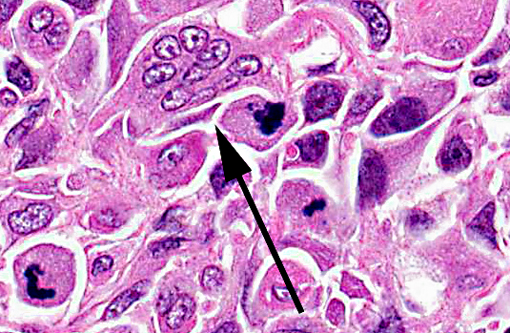
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Different subsets of OSA include those that produce osteoid (bone) (productive osteoblastic type), bone and cartilage (compound or combined type), are anaplastic with little extracellular osteoid production (poorly differentiated type), or rarely may form blood filled cysts lined by malignant osteoblasts (telangiectatic type).(2,5) Of these, osteoblastic (progressing to anaplastic) and combined type OSAs have been previously reported in rhesus macaques.(1,3) Based on the paucity of reported cases, little is known about the prognosis of these bony tumors in non-human primates.Â
Differential diagnoses for the grossly lytic lesion included bacterial or fungal osteomyelitis, which was deemed less likely given the radiographic evidence of pulmonary nodules. Specific infectious agents reported to cause osteomyelitis in non-human primates include Salmonella enteritidis, Mycobacterium tuberculosis, Coccodiodes immitis, and Histoplasma duboisii.(4) In addition, other neoplasms such as multiple myeloma, chondroblastoma, or other metastatic tumors could be considered. In this case, the bone biopsy revealed only reactive bone and was uninformative in distinguishing between neoplastic and inflammatory causes of bony lysis; this is a common issue with non-diagnostic specimens that miss the primary lesion.Â
Specific predisposing genetic mutations for the development of OSAs have not been reported in non-human primates, but several have been identified in dogs and humans. These include hereditary mutation in retinoblastoma (Rb) as well as sporadic mutations in p53, both common tumor suppressor genes. Mutations leading to overexpression of the p53 ubiquitin ligase MDM2 or decreased expression of the p14 gene product lead to increased p53 degradation and have been associated with OSA development. In addition, alterations in other genes include HER2/neu overexpression correlated with decreased survival and RECQL4 in the human Rothmund-Thomson Syndrome.(6)
JPC Diagnosis:
Conference Comment:
Conference participants compared and contrasted OSA in primates and dogs, with the following table provided by the conference moderator:
| Primates | Dogs | |
| Occurrence | Rare | Common |
| Age | Young | Old |
| Sites | Away from the elbow and towards the knee | Away from the elbow and towards the knee, except distal tibia > proximal* |
| Prognosis | 87% survival | Dismal |
*The moderator stressed an important caveat to the popular memory device away from the elbow and towards the knee (i.e., proximal humerus, distal radius, distal femur, proximal tibia) for common sites for canine OSA, noting that the distal tibia is actually more commonly affected than the proximal tibia.(2)
Discussing hereditary predispositions for developing osteosarcoma, the contributor brings up the interesting topic of tumor suppressor genes. Tumor suppressors such as RB and p53 are part of an intricate regulatory system in which they act to halt cellular proliferation in response to genetic damage. Active (i.e., hypophosphorylated) RB controls the cell cycle at the gap between mitosis and DNA synthesis (i.e., G1-S transition) by complexing with E2F transcription factors and recruiting chromatin-remodeling factors (e.g., histone deacetylases and histone methyltransferases), thereby inhibiting transcription of genes whose products are necessary for DNA synthesis in the S phase. When RB is inactivated via phosphorylation by the cyclin D-cyclin-dependent kinase (CDK) 4, cyclin D-CDK6 and cyclin E-CDK2 complexes, it releases E2F, which then activates the transcription of genes required for the S phase, and the cell cycle continues. Further regulation of this checkpoint occurs when growth inhibitors stimulate CDK inhibitors such as p16 (p16/INK4A) and p21 to inactivate the cyclin D-CDK complexes, leading to activated RB and subsequent cell cycle arrest. Thus, the G1-S checkpoint can be dysregulated by mutations in the genes that control the phosphorylation of RB: RB1, CDK4, and genes that encode cyclin D and p16.(7)
Tumor suppressor p53 is known as the guardian of the genome due to its critical role in regulating the cell cycle both at the G1-S and G2-M checkpoints. In healthy cells, p53 is quickly degraded by the ubiquitin pathway via MDM2; however, when DNA damage is sensed by ataxia-telangiectasia mutated (ATM) and ataxia-telangiectasia and Rad3 related (ATR) proteins, p53 undergoes post-transcriptional modifications causing it to be released from MDM2. Additionally, p14, another INK4A protein transcribed from the same gene as p16, has a protective effect on p53 by binding to and inhibiting MDM2. Once released from MDM2, p53 activates the transcription of numerous genes that cause cell cycle arrest and repair or apoptosis. Although p53 induces hundreds of genes, some of the key players include p21, which mediates cell cycle arrest by binding cyclin-CDK complexes and thus activating RB; GADD45, which aids in DNA repair; and BAX, which promotes apoptosis. If the DNA damage is successfully repaired, p53 up-regulates MDM2 transcription, which leads to its own destruction. If the DNA cannot be repaired, the cell becomes senescent (i.e., undergoes permanent, irreversible cell cycle arrest) or undergoes apoptosis. Similar to RB, mutations that cause loss of function in p53 or in the genes coding for proteins involved in the p53 pathway can disrupt this vital checkpoint and promote tumorigenesis.(7)
References:
2. Carlson CS, Weibrode SE. Bones, joints, tendons, and ligaments. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2012:959.
3. Gliatto JM, Bree MP, Mello NK. Extraosseous osteosarcoma in a nonhuman primate (Macaca mulatta). Journal of Medical Primatology. 1990;19(5):507-13.
4. Pritzker KPH, Kessler MJ. Arthritis, muscle, adipose tissue, and bone diseases of nonhuman primates. In: Abee CR, Mansfield K, Tardiff S, Morris T, eds. In: Nonhuman Primates in Biomedical Research, Vol. 2: Diseases. 2nd ed. St. Louis, MO: Elsevier; 2012:664.Â
5. Slayter MV, Boosinger TR, Pool RR, Dammarch J, Misdorp W, Larsen S. Histological Classification of Bone and Joint Tumors of Domestic Animals. World Health Organization. 2nd series. Vol. 1. Washington, DC: Armed Forces Institute of Pathology, American Registry of Pathology;1994.
6. Hansen MF. Genetic and molecular aspects of osteosarcoma. Journal of Musculoskeletal and Neuronal Interactions. 2002;2(6):554-560.
7. Stricker TP, Kumar V. Neoplasia. In: Kumar V, Abbas AK, Fausto N, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Saunders Elsevier; 2010:kindle edition, location 12860 of 92558.Â