WSC 22-23: Conference 1, Case 1
Signalment:
6-year-old, warmblood mare, equine (Equus caballus)
History:
The animal presented for treatment following a 24-hour history of lethargy and inappetence. The mare was diagnosed with strangles three weeks prior and was being medically managed with flunixin meglumine. At presentation, serum creatinine was 4.0 mg/dL, then rose to 10.2 mg/dL despite aggressive diuresis. The animal became anuric and was euthanized due to poor prognosis.
Gross Pathology:
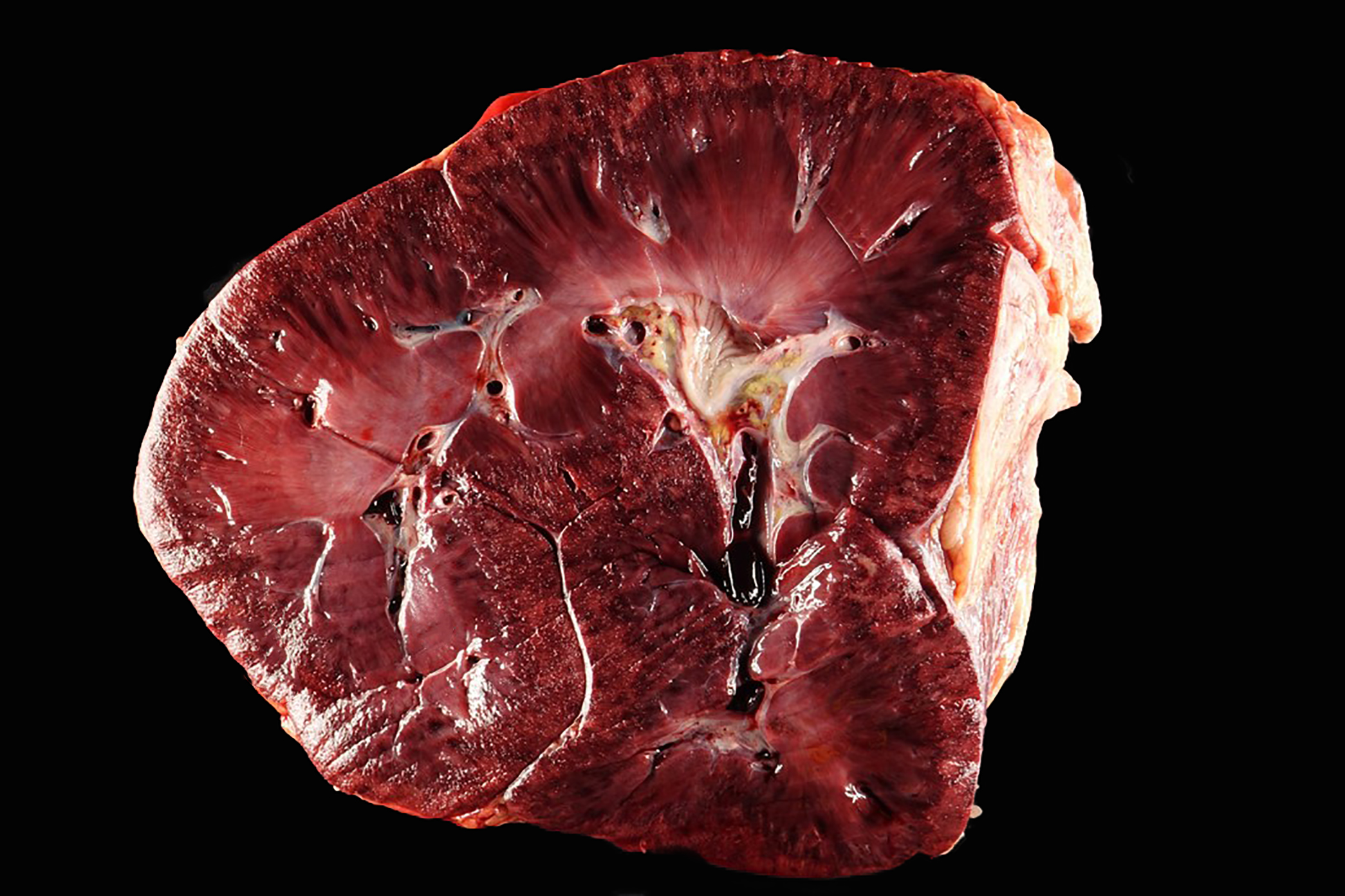
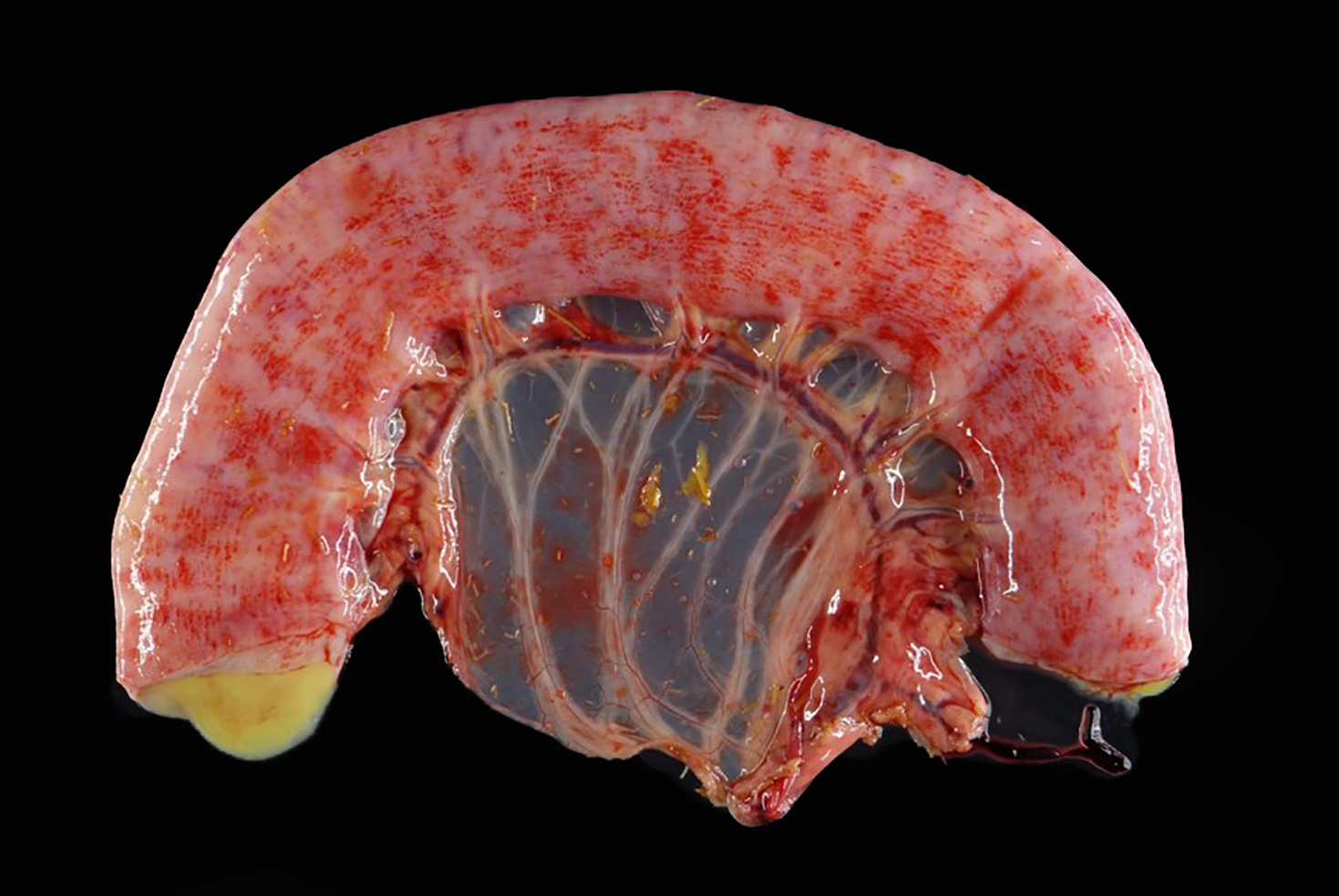
The kidneys were mottled dark red and tan throughout, with dark red to black streaks extending from the cortex into the medulla. Multiple abdominal serosal surfaces contained petechiae and ecchymoses. There was serosanguinous thoracic and pericardial effusion and generalized intramuscular and ventral subcutaneous edema. The mandibular and retropharyngeal lymph nodes were swollen, edematous and reddened, and at least one lymph node exuded purulent material when incised. One guttural pouch contained a hard nodule of inspissated purulent material adhered to the ventral mucosal surface.
Laboratory Results:
Aerobic culture from a retropharyngeal lymph node swab yielded moderate mixed growth including Streptococcus equi subsp. equi, Streptococcus equi subsp. zooepidemicus, Sphingobacterium spp. and Pseudomonas spp.
Microscopic Description:
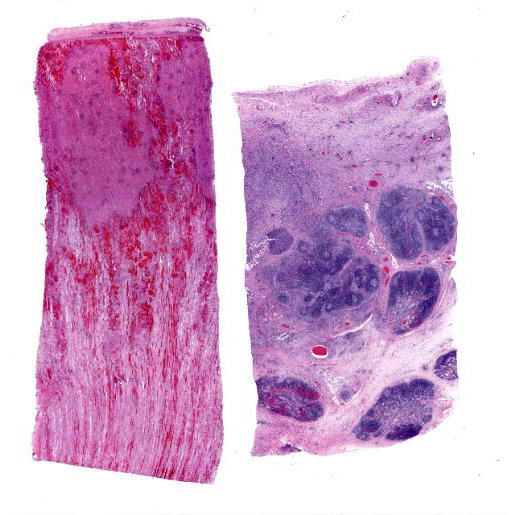
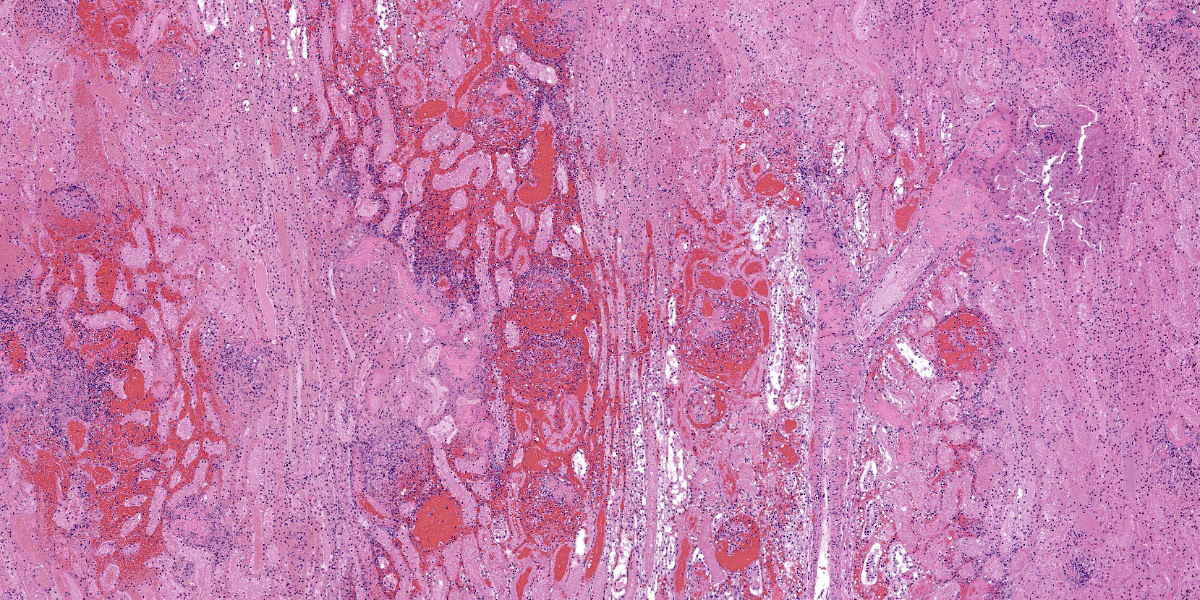
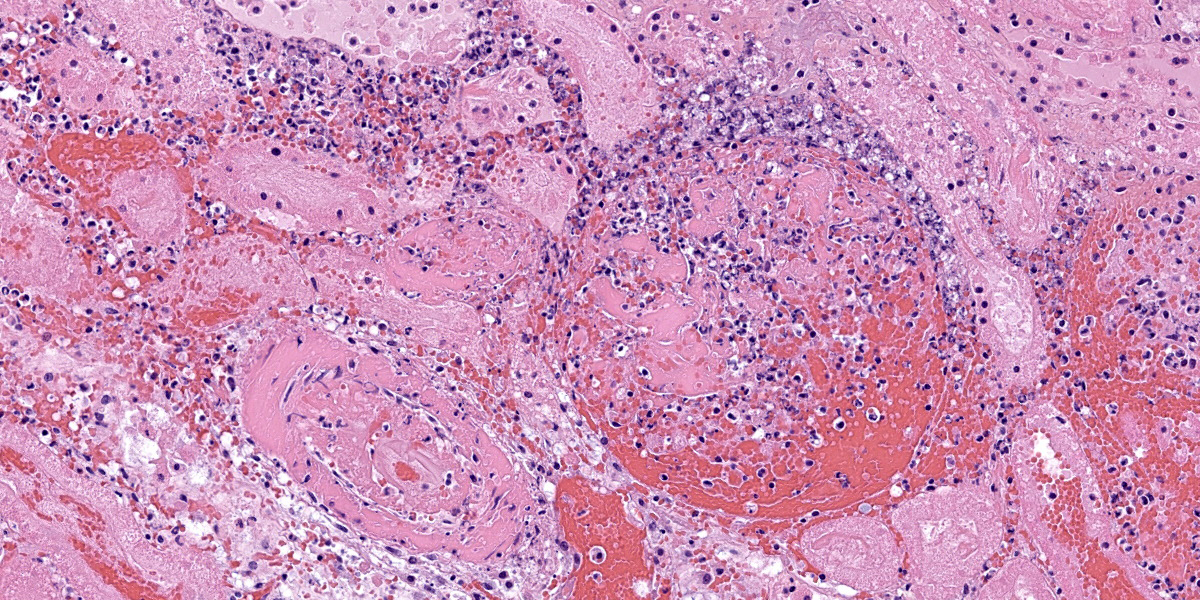
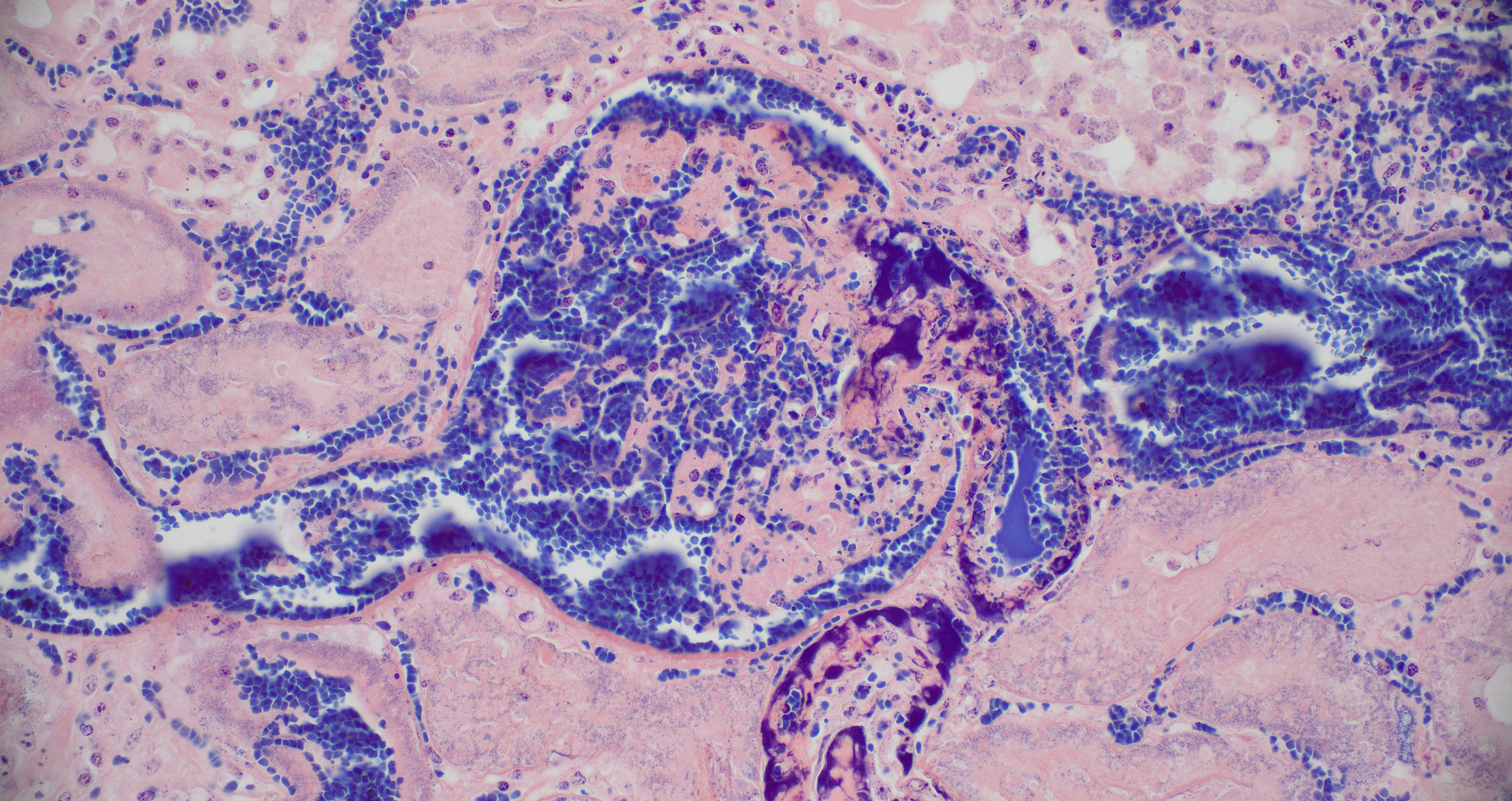
Kidney: Multiple irregular regions of the cortex are composed of coagulative necrosis characterized by partial loss of nuclear and cellular detail, hypereosinophilia, and karyorrhectic debris with maintenance of tissue architecture. Random scattered small to medium caliber blood vessels have segmental to circumferential transmural smudged eosinophilic walls infiltrated by intact and degenerate neutrophils, with some infrequent fragmentation of the tunica intima and media. Diffusely glomeruli in intact regions are segmentally to globally expanded by smudged homogenous eosinophilic matrix, intact and karyorrhectic neutrophils with fewer other mixed leukocytes (fibrinocellular crescents). Bowman’s space is frequently filled with erythrocytes. Tubular epithelial cells are multifocally pyknotic and sloughed into the lumen. The interstitium is multifocally expanded by small aggregates of neutrophils. Tubules are multifocally filled with brightly eosinophilic proteinaceous fluid. Adipose tissue on the capsular surface is infiltrated by lymphocytes, plasma cells, and neutrophils within a thin layer of well-vascularized fibrous tissue.
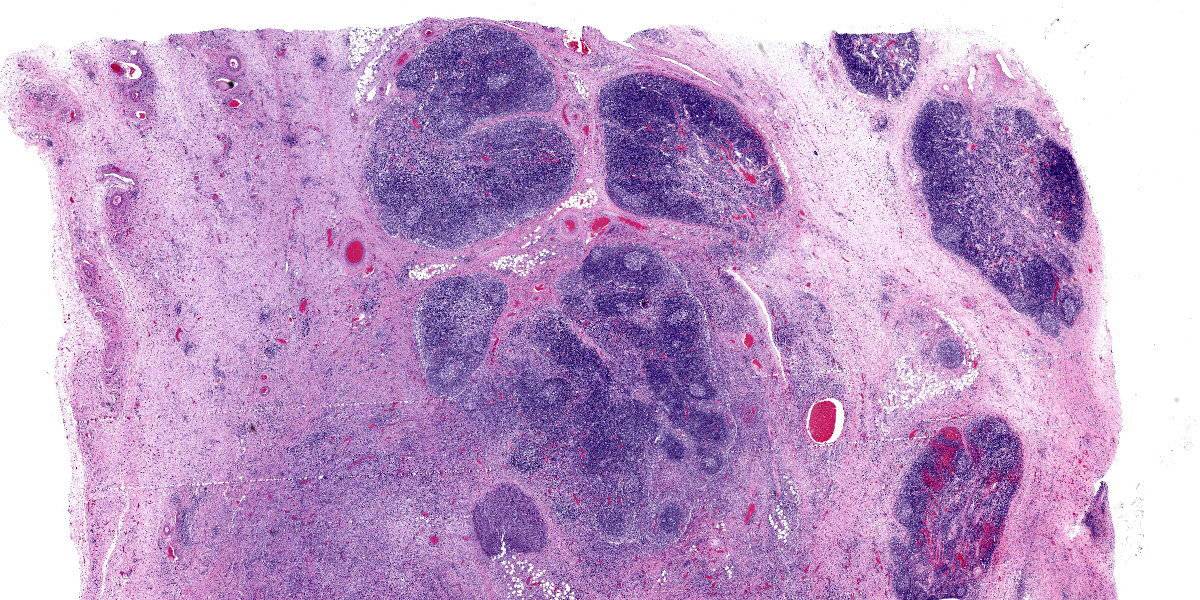
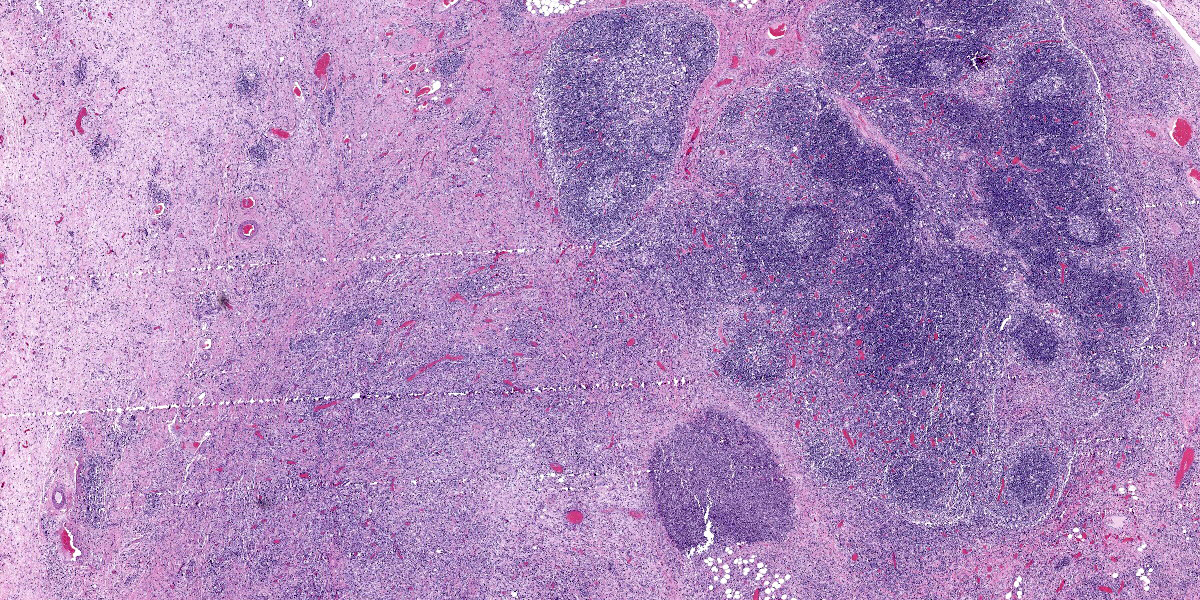
Lymph node: The architecture of the node is nearly effaced by extensive fibrosis admixed with aggregates of neutrophils which are occasionally ringed by epithelioid macrophages. Occasionally small clusters of basophilic 1-3 micron cocci are present within inflammatory foci. Lymphoid follicles are multifocally distributed through the tissue. Occasionally, small caliber blood vessels along the periphery are surrounded or obscured by moderate numbers of lymphocytes, plasma cells, neutrophils, and macrophages.
Contributor’s Morphologic Diagnoses:
Kidney: Glomerulonephritis, severe, multifocal, segmental to global, with fibrinocellular crescents.
Kidney: Fibrinonecrotizing and suppurative vasculitis, severe, multifocal subacute-active, with coagulative necrosis (infarction), hemorrhage and edema.
Lymph node: Pyogranulomatous lymphadenitis, severe, multifocal to coalescing, chronic, with fibrosis and rare cocci.
Contributor’s Comment:
Streptococcus equi equi-associated purpura hemorrhagica (PH) is a well-characterized, though uncommon immune complex-driven vasculitis following prolonged infection with S. equi equi, otherwise known as strangles. In the case of S. equi equi-associated PH, complexes are formed between Streptococcal M protein (SeM) and IgA which deposit in vessel walls.4 Immune complex formation and deposition in tissue depends on the amount of antigen, antigen:antibody ratio, and size of the complexes. Type III hypersensitivity reactions occur when there is slightly more antigen than antibody in circulation, forming complexes small enough to remain soluble but large enough to accumulate within tissue and initiate the complement cascade, resulting in the classic leukoclastic vasculitis.7,9 Other individual immunologic factors may also play a role in defective clearance of immunocomplexes. Free SeM can also activate the NLRP3 inflammasome, inducing IL-1β production, promoting pyroptosis in macrophages, and worsening systemic inflammation.8
Immune complexes can be detected in the circulation of affected horses.4 Horses with prior exposure or who are vaccinated are at a slightly higher risk of developing PH with subsequent S. equi equi infection, presumably due to preexisting antibody titers priming formation of immunocomplexes.1 The percent of horses with Strangles that develop PH varies, with two studies of outbreaks reporting 6.5% and 5.4% respectively.2 Other bacteria, including Corynebacterium pseu-dotuberculosis, and some viruses induce PH, also through a type III hypersensitivity mechanism.9
The typical clinical presentation of PH is variable but often includes well-demarcated, gravity-dependent edema with petechiae and/or ecchymoses on the mucous membranes and skin.1,10 This case is unusual inthat the primary clinical presentation was acute anuric renal failure, presumably secondary to the numerous and extensive renal infarctions. Infarctive PH resulting from vessel occlusion secondary to vasculitis is described most commonly with infarction of skeletal muscle, leading to stiffness and pain, or in the gastrointestinal tract, leading to colic. While the infarctions were responsible for acute severe renal failure, the non-infarcted glomerular tufts contained abundant inflammation, regions of necrosis, and fibrin thrombi (fibrinocellular crescents). Glomeruli are a well-known target for immunocomplex (IC) deposition in a variety of type III diseases such as systemic lupus erythematosus. Demonstration of glomerular IC deposition has not been confirmed in horses with S. equi equi associated PH, however glomerulonephritis with basement membrane IC deposition is reported as part of post-streptococcal infection in humans.6 Henoch-Schonlein syndrome is a human form of IgA IC disease that causes purpura, arthritis, gastrointestinal symptoms, and glomerulonephritis and is thought to be caused by infections including streptococcal organisms, viruses, medication, insect bites and other causes.5 It is suspected that the glomerulonephritis observed in this case may have been due to local IC deposition; however, the extensive tissue damage and inflammation precludes more careful evaluation of glomerular architecture and basement membrane thickness via electron microscopy. Special stains, including PTAH and PAS, confirmed fibrin deposition in glomeruli, though the mesangial basement membrane was predominantly obscured.
Contributing Institution:
Colorado State University
Veterinary Diagnostic Laboratory
https://vetmedbiosci.colostate.edu/vdl
JPC Diagnosis:
Kidney, vessels and glomeruli: Vasculitis, necrotizing, multifocal to coalescing, severe with thrombosis and extensive cortical and medullary infarction.
Lymph node: Lymphadenitis, suppurative, focally extensive, moderate, with reactive hyperplasia.
JPC Comment:
Purpura hemorrhagica is a prototypical Type III hypersensitivity-mediated disease. As the contributor notes, the classic histologic lesion is leukoclastic vasculitis, with an inflammatory infiltrate composed of neutrophils in which nuclei have disintegrated into fragments termed “nuclear dust” or, less poetically, “leukocytoclasia.”3 The contributor provides a nice discussion of the factors that lead to immune complex deposition within vascular walls and trigger the Type III hypersensitivity reaction.
Type III hypersensitivity reactions can be thought of as “innocent bystander” reactions because the injured tissue is not a direct target of the immune response; rather, once deposited within tissues, the immune complexes themselves activate multiple cellular processes and cascades that result in inflammation and tissue damage. 7 Most critically, immune complexes lead to complement activation when IgG and/or IgM are cross-linked with C1, leading to the formation of the C3 and C5 convertases of the classical complement pathway. Cleavage products of classical pathway, C3a and C5a, cause increased vascular permeability and vasodilation. C5a is also chemotactic for neutrophils and macrophages, luring them to sites of immune complex deposition where their released and elaborated proteolytic enzymes and free radicals damage surrounding tissues. Vascular damage can compromise the intima, leading to exposure of subintimal collagen, coagulation cascade and platelet activation, and the production of microthombi with subsequent infarction.
This sequence of Type III hypersensitivity-mediated injury is not unique to Strangles-associated purpura hemorrhagica, but is part of a stereotyped pattern of injury in several immune-mediated diseases of veterinary importance. The contributor mentions systemic lupus erythematosus, where DNA and nuceloproteins serve as antigenic niduses for immune complex formation. Others include equine infectious anemia, equine recurrent infectious uveitis, “blue eye” secondary to canine adenovirus 1 infection, hypersensitivity pneumonitis, rheumatoid arthritis, and acute glomerulonephritis, among others.7
The conference moderator, COL Jeremy Bearss, outgoing JPC director, stressed the importance of examining vessels in all tissues. Though easily overlooked among more eye-catching histologic features, the vessels often contain a wealth of diagnostic information as in this case, where the leukoclastic vasculitis is a key histologic feature.
Careful consideration was given to the contributor’s description of fibrinocellular crescents; however, no fibrinocellular crescents were noted in the histologic sections examined during the conference.
References:
- Boyle AG, Timoney JF, Newton JR, Hines MT, Waller AS, Buchanan BR. Streptococcus equi infections in horses: guidelines for treatment, control, and prevention of Strangles—revised consensus statement. J Vet Intern Med. 2018;32(2):633-647.
- Duffee LR, Stefanovski D, Boston RC, Boyle AG. Predictor variables for and complications associated with Streptococcus equi subsp equi infection in horses. J Am Vet Med Assoc. 2015;247 (10):1161-1168.
- Fraticelli P, Benfaremo D, Gabrielli, A. Diagnosis and management of leukocytoclastic vasculitis. Intern Emerg Med. 2021;16:831-841.
- Galan JE, Timoney JF. Immune complexes in purpura hemorrhagica of the horse contain IgA and M antigen of Streptococcus equi. J Immunol. 1985; 135(5):3134-3137.
- Lanzkowsky S, Lanzkowsky P, Schoenlein J. Henoch-Schoenlein Purpura. Pediatr Rev. 1992;13(4).
- Rodriguez-Iturbe B, Haas M. Post-Streptococcal Glomerulonephritis. In: Ferretti JJ, Stevens DL, Fischetti VA, eds. Streptococcus pyogenes: Basic Biology to Clinical Manifestations. University of Oklahoma Health Sciences Center; 2016.
- Snyder PW. Diseases of Immunity. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. Elsevier; 2017:242-285, 319-322.
- Valderrama JA, Riestra AM, Gao NJ, et al. Group A streptococcal M protein activates the NLRP3 inflammasome. Nat Microbiol. 2017;2(10):1425-1434.
- Valentine BA. Skeletal Muscle. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. Elsevier; 2017:908-953.
- Whelchel DD, Chaffin MK. Sequelae and complications of Streptococcus equi subspecies equi infections in the horse. Equine Vet Educ. 2009;21(3):135 -141.