WSC 2023-2024, Conference 21, Case 2
Signalment:
13-year-old, female spayed Yorkshire terrier (Canis familiaris)
History:
A 13-year-old Yorkshire terrier presented with a previously debulked oral mass associated with the caudal hard palate and soft palate. After 3 months of palliative treatment, the dog presented with inappetence, weight loss, and increased respiratory effort. Physical examination showed rapid growth of the mass since the most recent checkup, with partial upper airway obstruction. Euthanasia was elected given the rapid clinical decline.
Gross Pathology:
Within the caudal oral cavity and oropharynx, there is an irregularly shaped, 5 x 3 x 1.5 cm, firm, pale tan to dark gray mass extending ventrally from the hard and soft palate. The submandibular lymph nodes are enlarged, firm, and gray on cut section with loss of corticomedullary architecture. There are dozens of, firm, pale tan to gray, round to multinodular masses ranging from 2-10 mm in diameter within the lungs, mediastinum, spleen, liver, and right adrenal gland.
Microscopic Description:
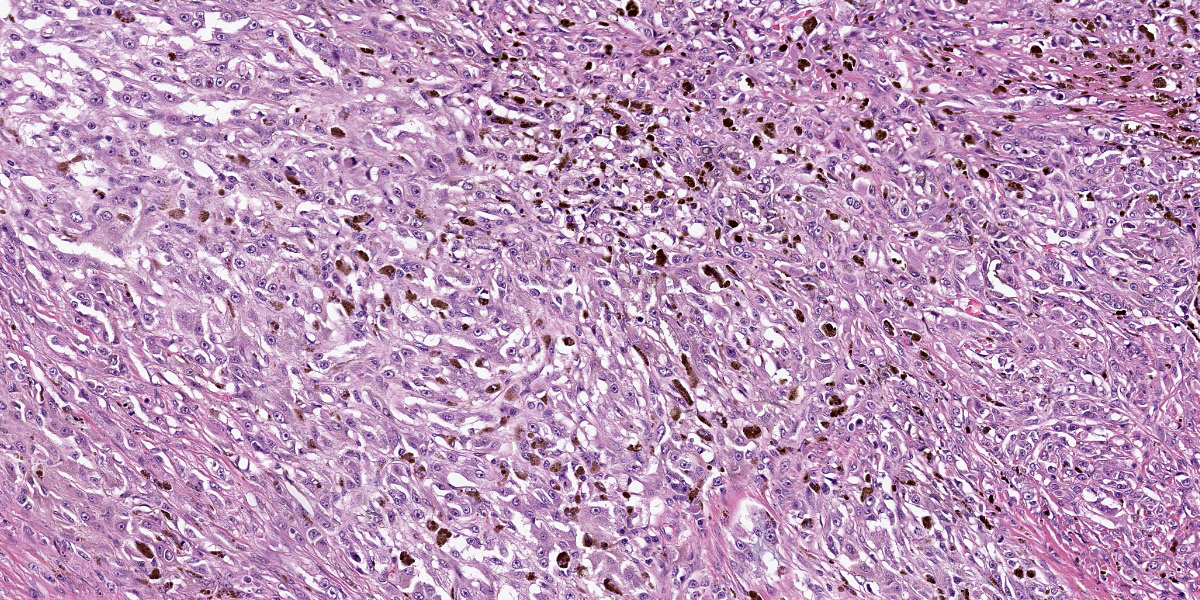
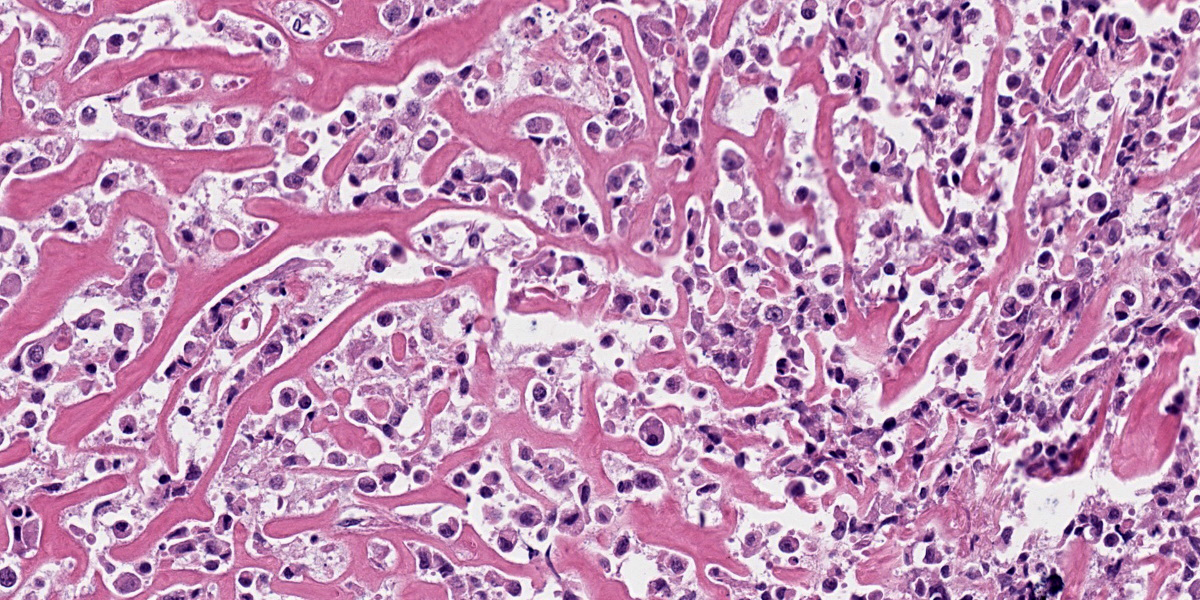
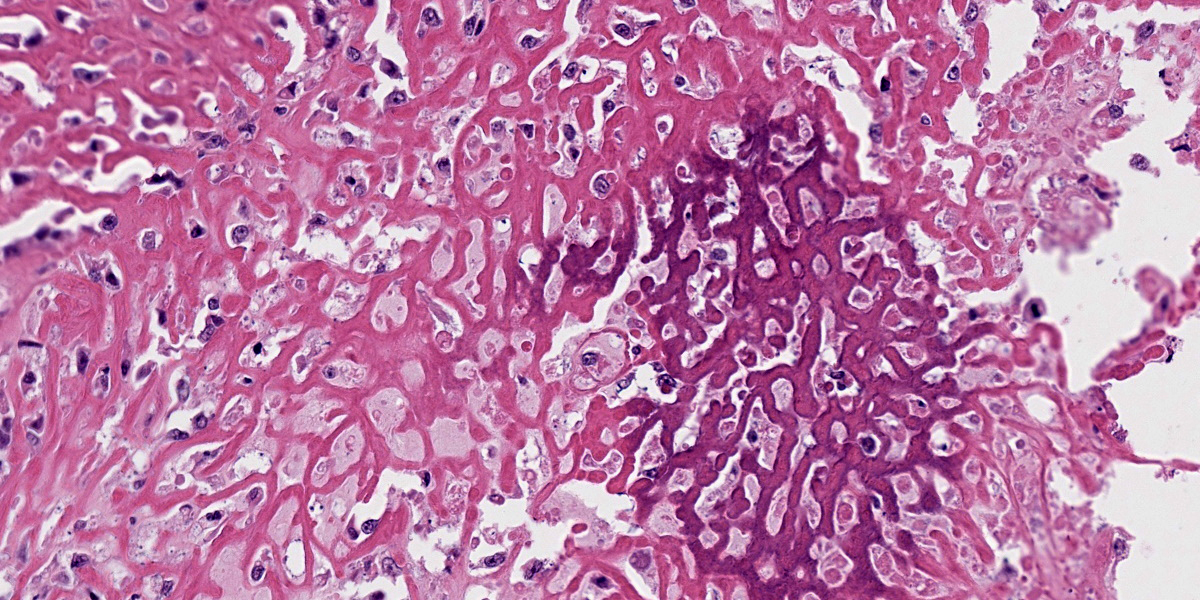
Oral mucosa: Expanding the lamina propria is an unencapsulated, highly cellular mass composed of a pleomorphic population of neoplastic cells supported by sparse fibrovascular stroma. Neoplastic cells range from polygonal and arranged in sheets or thin cords, to spindle-shaped and arranged in streams and bundles. The neoplastic cells have variably distinct borders, abundant eosinophilic cytoplasm, round to ovoid nuclei, finely stippled chromatin, and 1-2 prominent nucleoli. Anisocytosis is marked and anisokaryosis is moderate. There are 24 mitotic figures in 10 examined 400X fields. Rarely, neoplastic cells contain scant black, fine, granular intracytoplasmic material (melanin). Sporadically throughout the mass, there are numerous round cells with abundant black, granular intracytoplasmic material (melanomacrophages). Within the neoplasm are several foci of homogenous, eosinophilic, extracellular matrix (osteoid) that is frequently associated with individual or thin aggregates of neoplastic cells, and rarely mineralized. Adjacent to and entrapped within the neoplasm are occasional islands of salivary gland tissue and salivary ducts. Centrally within the mass, there are large foci of coagulation necrosis with loss of differential staining, pyknotic and karyorrhectic debris, and occasional pockets of neutrophils. The overlying mucosa is multifocally ulcerated and replaced by a mat of fibrin and necrotic cellular debris with abundant bacteria of mixed morphology.
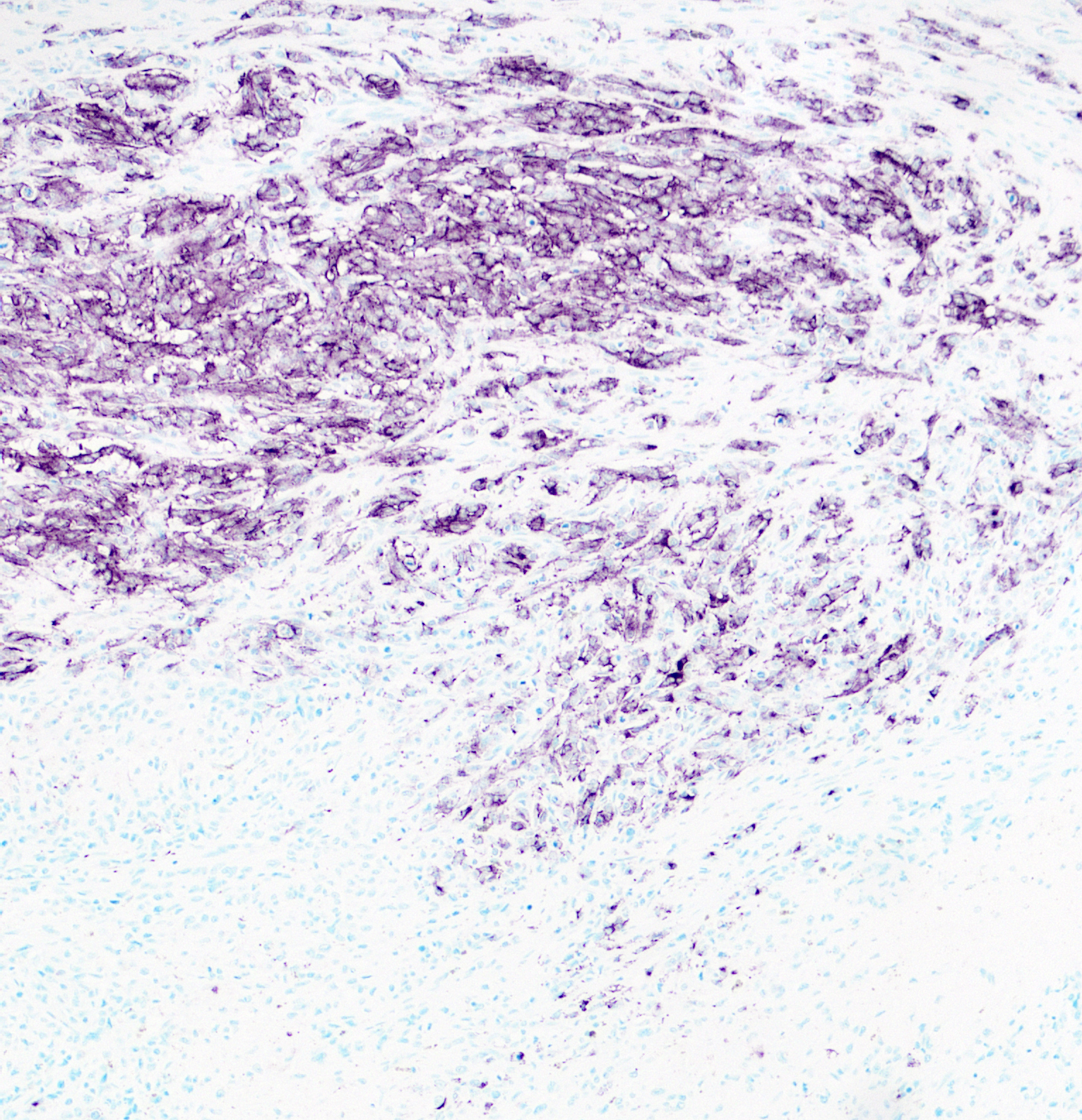
Immunohistochemistry for SOX10, PNL2, Melan-A, and S100 was performed. >80% of neoplastic cells exhibit strong, nuclear labeling for SOX10 and cytoplasmic labeling for S100. Approximately 50% of neoplastic cells exhibit cytoplasmic labeling for PNL2 with regional variability throughout the tissue. Only sparse neoplastic cells exhibit strong cytoplasmic labeling for Melan-A (<10%).
The same neoplastic population with variable deposition of osteoid comprises all masses in other examined tissues (metastatic sites).
Contributor’s Morphologic Diagnosis:
Oral mucosa: Malignant melanoma, osteogenic.
Contributor’s Comment:
Malignant melanoma, composed of neoplastic melanocytes of neural crest ectoderm origin, is one of the most common oral tumors in dogs.16 Canine oral melanomas have been associated with worse clinical outcomes than those arising at other sites. Most canine oral melanomas have metastasized by time of diagnosis, and the median survival time with treatment is under 3 years.7,9,16 However, a subset of oral melanocytic tumors in dogs exhibits a less aggressive biological behavior despite being characterized histologically as malignant.5 Mitotic index, nuclear atypia, and Ki67 positivity are reported as relevant prognostic features.14 Oral melanomas frequently contain a significant amelanotic component, as in this case, but this does not correlate well with biological behavior.
Melanomas can vary widely in their histologic appearance. Cellular morphology can range from round to polygonal to spindle-shaped, often within the same anaplastic tumor, likely reflecting their neural crest origin drawing from both neural and epithelial roots. Junctional activity (subepithelial proliferation of neoplastic cells) is considered a characteristic feature that should increase suspicion for melanoma, even when pigmentation is sparse or absent.
The presence of osteoid in the stroma of this melanoma is a rare concurrent finding in canine melanomas, with only a few case reports in the literature.3,4,8,11 While this osteogenic variant of melanoma is rare, it is important to recognize since osteosarcoma and malignant melanoma differ in prognosis and treatment. Human cases of melanoma with stromal osteoid and/or bone are also rare.12 Heterologous differentiation in human melanoma is well-recognized and characterized, although these patterns are not widely used in veterinary species and prognostic significance has not been established. Such differentiation patterns include osteocartilaginous, fibroblastic, rhabdomyoblastic, Schwannian, and ganglionic.1 Cases with osteoid and bone formation are classified as “osteosarcomatous” or “osteocartilaginous” which, as implied by the names, frequently also contain cartilage.2,6,10
The mechanism for osteoid and bone formation with canine or human melanoma is unresolved, but multiple hypotheses have been proposed.1 Because some cranial skeletal elements are derived from neural crest, some postulate that metaplasia of pluripotent precursors results in production of osteoid and bone by the tumor cells. Others have speculated that it represents a stromal response to local trauma, although this does not explain production at sites of metastasis. Alternatively, osteoid and bone could be produced by the tumor stroma in response to release of factors by the neoplastic cells. Such factors have not been characterized in cases of osteogenic melanoma, but could include bone morphogenetic proteins, as an example. Of these possible sources, direct production by metaplastic tumor cells is favored in this case since the matrix is directly surrounded by cells that are morphologically consistent with the neoplastic population; however, immunohistochemistry may contradict this interpretation since most of these “osteogenic” cells are negative for our melanocytic markers.
Because anaplastic melanomas can pose a diagnostic challenge, particularly with poor pigmentation and divergent differentiation, immunohistochemical evaluation is a useful tool. Markers for which melanocytic tumors are typically positive include Melan-A, PNL2, S100, SOX10, TRP-1, and TRP-2.13,15 Over-reliance on a single immunohistochemical marker can result in false negatives. This occurred in the present case, as the top clinical differential was a poorly-differentiated sarcoma based on a reported negative result for Melan-A on the initial mass debulk.
Contributing Institution:
The Ohio State University
College of Veterinary Medicine
Department of Veterinary Biosciences
https://vet.osu.edu/biosciences
JPC Diagnosis:
Oral mucosa: Osteogenic melanoma.
JPC Comment:
As noted by the contributor, osteogenic melanoma is rare in both human and veterinary medicine. Due to the paucity of reported cases, it is unclear if cartilaginous or osteogenic differentiation is of prognostic significance; therefore, current prognoses and treatment plans for these neoplasms are the same as for conventional, non-productive melanomas.4
Most reported cases of osteogenic melanoma have occured primarily in older, small breed dogs, are typically oral, and arise primarily in the gingiva.4 As noted above, the biological behavior of these tumors is, in the small number of reported cases, similar to oral melanoma.4 Features common to reported cases of osteogenic melanoma are the absence of primary bony involvement, the presence of junctional activity, and neoplastic cells with distinct melanin granules or Melan-A and S-100 positive cells adjacent to and/or embedded in tumor osteoid.4
While the molecular basis for the osteogenic part of osteogenic melanoma remains unclear, melanomas are known to express “runt-related transcription factor 2,” or RUNX2, an osteogenic master regulator typically only expressed in osteoblasts and in mesenchymal stem cells during osteogenic commitment.2,17 In vitro studies have shown that melanoma cells can be induced to form bone directly by expression of both bone sialoprotein and RUNX2.4 Increased RUNX2 expression also induces the expression of specific metalloproteinases and collagenases that potentiate matrix degradation and tumor invasion. Because of this, it has been suggested that the presence of neoplastic bone in malignant melanoma suggests that mesenchymal conversion has occurred and that osteogenic melanoma may, therefore, have a greater metastatic potential when compared to its non-osteogenic counterpart.4
The presence of osteoid, multinucleated cells, and suspected melanin-containing cells initially stymied diagnostic progress among conference participants, and discussion quickly narrowed to the two main differentials of melanoma and osteosarcoma. Further frustrating efforts was the paucity of oral mucosa in the examined section, making it difficult to assess junctional activity, the presence of which would have helped convince a skeptical audience of the melanocytic origin of this neoplasm. Conference participants discussed and reviewed melanocytic immunohistochemical marker PLN2, which was convincingly positive in approximately 30-40% of neoplastic cells. Participants remarked on the quantitative and qualitative variability in staining among the neoplastic cells, causing the moderator to speculate that not all neoplastic cells are perfectly clonal, and this variability in gene expression, coupled with genetic variability induced by the tumor microenvironment could account for the pleomorphic quality often seen with immunohistochemical staining.
Conference participants discussed whether the prominent bone within section was produced by the neoplasm, or part of normal anatomic structures. The moderator noted that the section was not decalcified, which would normally be required for section through the mandible or maxilla, suggesting that all the bone in section was produced by the tumor.
The moderator cautioned participants to maintain an index of suspicion for osteogenic melanoma, particularly in the oral cavity where neoplasms such as ossifying fibroma and osteosarcoma or lesions producing cemento-osseous matrix are more apt to come to mind when presented with spindle cells producing an osteoid-like matrix.
References:
- Banerjee SS, Eyden B. Divergent differen-tiation in malignant melanomas: a review. Histopathology. 2008;52(2):119-129.
- Barroso de Carvalho B, Batista Dos Santos Medeiros D. Melanoma with osteo-cartilaginous differentiation. An Bras Dermatol. 2023;98(2):266-269.
- Chénier S, Doré M. Oral malignant melanoma with osteoid formation in a dog. Vet Pathol. 1999;36(1):74-76.
- Ellis AE, Harmon BG, Miller DL, North-rup NC, Latimer KS, Uhl EW. Gingival osteogenic melanoma in two dogs. J Vet Diagn Invest. 2010;22(1):147-151.
- Esplin DG. Survival of dogs following surgical excision of histologically well-differentiated melanocytic neoplasms of the mucous membranes of the lips and oral cavity. Vet Pathol. 2008;45(6):889-896.
- Lucas DR, Tazelaar HD, Unni KK, et al. Osteogenic melanoma. A rare variant of malignant melanoma. Am J Surg Pathol. 1993;17(4):400-409.
- Nishiya AT, Massoco CO, Felizzola CR, et al. Comparative aspects of canine melanoma. Vet Sci. 2016;3(1):7.
- Oyamada T, Tanaka H, Park C-H, Ueki H, Komiya T, Arai S. Pathology of canine oral malignant melanoma with cartilage and/or osteoid formation. J Vet Med Sci. 2007;69(11):1155-1161.
- Pazzi P, Steenkamp G, Rixon AJ. Treatment of canine oral melanomas: a critical review of the literature. Vet Sci. 2022;9(5):196.
- Saleh J, Wang ML, Harms PW, Patel RM, Fullen DR. Malignant melanoma with osteosarcomatous differentiation in a lymph node metastasis. J Cutan Pathol. 2018;45(9):701-704.
- Sánchez J, Ramirez GA, Buendia AJ, et al. Immunohistochemical characterization and evaluation of prognostic factors in canine oral melanomas with osteo-cartilaginous differentiation. Vet Pathol. 2007;44(5):676-682.
- Savant D, Kenan S, Kenan S, Kahn L. Osteogenic melanoma: report of a case mimicking osteosarcoma and review of the literature. Skeletal Radiol. 2018;47(5): 711-716.
- Smedley RC, Lamoureux J, Sledge DG, Kiupel M. Immunohistochemical diag-nosis of canine oral amelanotic melan-ocytic neoplasms. Vet Pathol. 2011;48(1): 32-40.
- Smedley RC, Spangler WL, Esplin DG, et al. Prognostic markers for canine melan-ocytic neoplasms: a comparative review of the literature and goals for future inves-tigation. Vet Pathol. 2011;48 (1):54-72.
- Tsoi MF, Thaiwong T, Smedley RC, Noland E, Kiupel M. Quantitative exp-ression of TYR, CD34, and CALD1 discriminates between canine oral mal-ignant melanomas and soft tissue sarcomas. Front Vet Sci. 2021;8:701457.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Vol 2. Saunders;2016:1-257.e2.
- Valenti MT, Carbonare LD, Mottes M. Ectopic expression of the osteogenic master gene RUNX2 in melanoma. World J Stem Cells. 2018;10(7):78-81.