WSC 2023-2024 - Conference 1, Case 2
Signalment:
1-month-old, male, Rasa Aragonesa sheep (Ovis aries)
History:
This animal was one of 400 lambs on a sin-gle farm. 250 lambs became lethargic and apathetic and 10 lambs died suddenly.
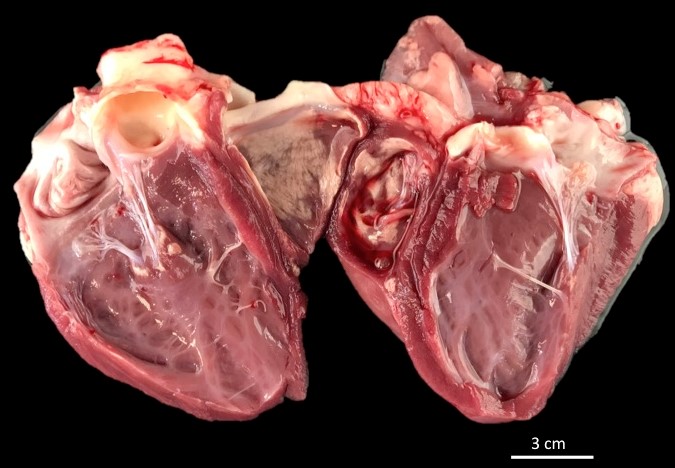
Gross Pathology:
Approximately 40% of the endocardium and myocardium of both ventricles had well-demarcated, multifocal foci of white, hard, crunchy tissue admixed with areas of mild hemorrhage.
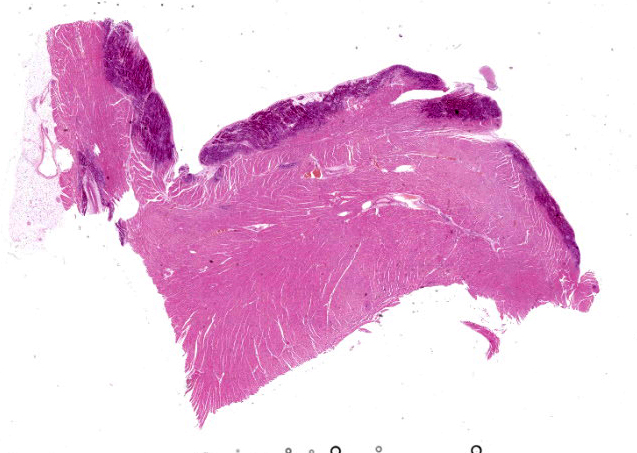
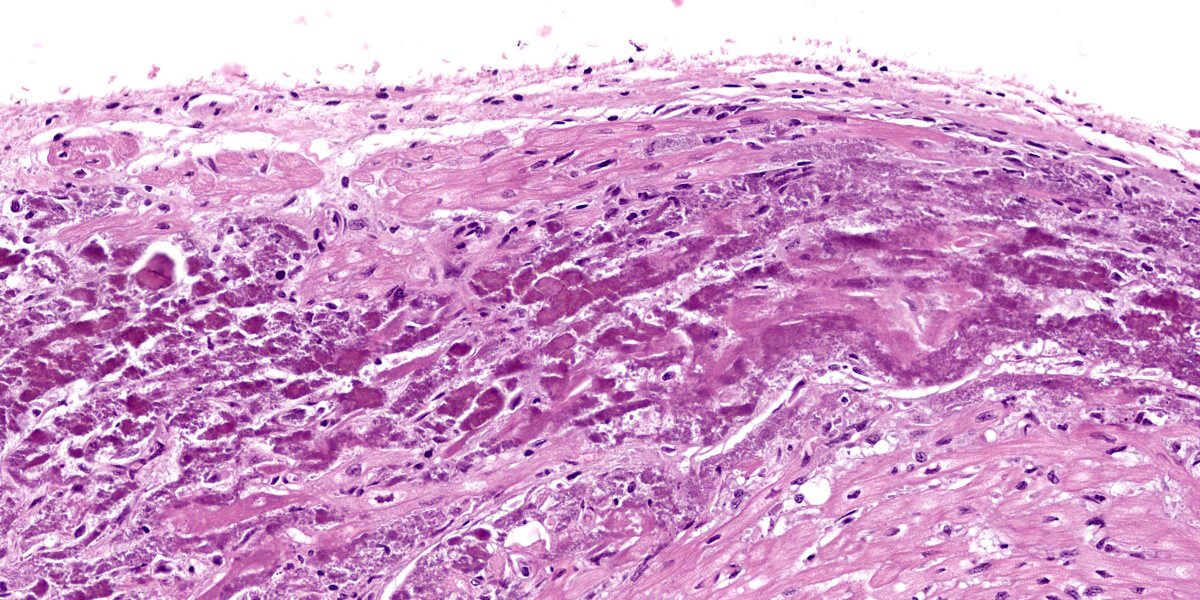
Microscopic Description:
Heart: Diffusely up to 40% of the tissue is affected by a degenerative and necrotizing process. On a focally extensive area of the myocardium close to endocardium, some cardiomyocytes are swollen with moderate amounts of vacuolation and pale eosino-philic cytoplasm (degeneration), others are shrunken and hypereosinophilic, with loss of cross striations and fragmentation of myofi-brils, and occasional contraction bands, with pyknosis and karyorrhexis (necrosis). Many degenerate and necrotic myocytes contain abundant amounts of basophilic granular material (mineral). Multifocally, scattered among the tissue are few neutrophils and lymphocytes admixed with moderate edema and mild hemorrhage. Few satellite nuclei surrounding affected fibers are hyperplastic.
Contributor’s Morphologic Diagnosis:
Cardiac muscle: Focally extensive necrosis and degeneration with mineralization, poly-phasic, subacute, moderate.
Contributor’s Comment:
The most common nutritional deficiency leading to nutritional myopathy is selenium deficiency. Nutritional myopathy resulting from vitamin E deficiency in the absence of selenium deficiency is uncommon in mam-mals but may be more common in birds and reptiles.2 Selenium is primarily considered a powerful antioxidant because of its role in the glutathione peroxidase system.4 Antioxi-dant systems are believed to have evolved as a means of surviving in an oxygenated at-mosphere by dealing with free radicals and the toxic products of their metabolism. An-imal antioxidant defense mechanisms are based on the synthesis of numerous biologi-cal antioxidants that include the antioxidant enzymes glutathione, thioredoxin, and coen-zyme Q. There is also a range of dietary an-tioxidants which can be provided in feed, which include vitamin E, carotenoids, poly-phenolics, and selenium (as a precursor to selenoproteins). Under stress conditions, the internal antioxidant system network alone cannot deal properly with excess reactive oxygen species formation and requires addi-tional help from dietary antioxidant sources provided via feed/water. Vitamin E and se-lenium are major feed-derived antioxidants.8
Nutritional myopathy in sheep is probably more prevalent in more areas of the world than the disease in cattle. The names white muscle disease, rigid lamb disease, and stiff lamb disease were coined to describe the most frequently encountered clinical pat-terns in 2-4 week-old lambs, which very of-ten are spring lambs, recently turned out on-to the first green pasture.2 Congenital nutri-tional myopathy does occur in lambs, but not often. The typical disease may occur as an outbreak among lambs from 1 day to 2 months of age or beyond. Mortality at this stage may be very low or may reach 50%. The next peak of incidence occurs at 4-8 months of age as weaned lambs are put onto lush pastures following mowing or into feedlots. Mortality is not usually very high, but the incidence of minimal clinical disease may be moderately high and that of subclin-ical disease may be higher still.2
Lesions and their corresponding clinical signs are as varied as the circumstances un-der which myopathy occurs. The lesions may be detectable in lamb fetuses at least 2 weeks before parturition. In the congenital disease, tongue and neck muscles used in suckling movements often contain the most severe lesions. When the lesions occur in lambs a few days older, they are likely to be much more extensive and involve primarily the major muscles of the shoulder and thigh but also back, neck, and respiratory (dia-phragm and intercostal) muscles.2 Severe, often fatal myocardial necrosis is typically part of the important vitamin E and seleni-um-responsive syndromes of nutritional my-opathy of lambs, calves, swine, and horses,8 and in mulberry heart disease of swine. It may also be seen as part of equine rhabdo-myolysis and of capture myopathy and other exertional syndromes where vitamin E and selenium depletion produce cardiomyocyte alterations characterized by contraction band necrosis and myofibrillar lysis.4,7
Given the potential for a common mecha-nism of cell injury in both selenium defi-ciency and selenium toxicity, it is not sur-prising that the primary lesion in these two diseases is myocardial necrosis. Therefore, in cases of acute myocardial necrosis, it is imperative that tissue selenium levels are quantified before a diagnosis of selenium deficiency is rendered.1 Polyunsaturated fat-ty acid levels must also be examined as sup-plementation with polyunsaturated fatty ac-ids can greatly increase the severity of these changes.4
Several mineral deficiencies might be pre-sent at the same time in a sheep herd. Cases of poor growth performance in lambs should be investigated taking several mineral defi-ciencies, particularly cobalt, copper, and selenium into account. Clinical examination can often give only suspected diagnoses, so to access possible mineral deficiencies, a nutritional assessment should be performed sampling not only blood, but also liver tissue via biopsy or post-mortem samples.3
Contributing Institution:
University of Zaragoza
Departamento de Patología Animal (Área histología)
University of Zaragoza
Zaragoza, Spain
JPC Diagnosis:
Heart, myocardium: Mineralization and ne-crosis, subendocardial, diffuse, severe, with fibrosis.
JPC Comment:
The striking myocardial mineralization ob-served grossly and histologically in this case is an excellent example of pathologic calci-fication. Pathologic calcification is the ab-normal deposition of calcium salts, along9 with smaller amounts of iron, magnesium, and other mineral salts, within soft tissues.6 Pathologic calcification comes in two forms: dystrophic calcification and metastatic calcification. Metastatic calcification occurs primarily in normal tissues due to a calcium-phosphate imbalance (“metastatic is metabolic”) and is more likely to occur when the serum product of the calcium-phosphorus concentration exceeds 70 mg/dL. Causes for calcium-phosphate imbalance are many and include chronic kidney disease, vitamin D toxicosis, inappropriately elevated parathyroid hor-mone, or humoral hypercalcemia of malig-nancy. Metastatic calcification tends to oc-cur in tissues that exist in alkaline environ-ments, primarily the stomach, kidneys, and lungs.
Dystrophic calcification, by contrast, occurs in areas of tissue necrosis (“dystrophic is dead”) and typically occurs in patients with normal serum calcium levels. Intracellular calcium overload is an expected conse-quence of cell death as ischemia leads to the opening of membrane calcium channels and the flooding of the cytosol with calcium normally sequestered in the sarco- or endo-plasmic reticulum and mitochondria.5 Nec-crotic myofibers are particularly prone to dystrophic calcification due to the high lev-els of calcium ions stored in the sarcoplas-mic reticulum; thus, selenium or vitamin E deficiency-driven necrosis may quickly lead to whole myocyte calcification and to the white, gritty lesion for which white muscle disease gets its common name.5
Nutritional myopathy, the preferred term for white muscle disease, may present different-ly in different species. The contributor pro-vides a good summary of the typical disease presentation in young, suckling animals. In affected adult horses, the temporal and mas-seter muscles are often preferentially affect-ed with swelling and stiffness leading to im-paired mastication.9 The disease in cattle, sheep, goats, and camelids more typically affects the postural muscles and the muscles of locomotion without the profound in-volvement of the temporalis and masseter muscles seen in horses.9 Severe disease in all species can lead to necrosis and dys-trophic calcification of the myocardium or endocardium, as illustrated in this case.
Lesions may also differ based on chronicity. In animals with severe, acute myopathy leading to death (typically young animals) lesions are characterized by widespread muscle necrosis and dystrophic mineraliza-tion with minimal inflammation. In subacute or chronic disease, lesions are polyphasic with active necrosis, macrophage infiltra-tion, and regeneration all present simultane-ously.9
There was dispirited discussion among con-ference participants about the phasic nature of the myocardial lesions in this case. Le-sions resulting from nutritional myopathy, a chronic process, would be expected to be polyphasic; however, the slides reviewed in conference appeared to the moderator and conference participants to be largely mo-nophasic and characterized solely by necro-sis and mineralization. Participants dis-cussed whether the young age of the animal at death precluded full lesion development or if the examined histologic section was unrepresentative. No satisfactory resolution was reached during conference discussion and the final JPC morphologic diagnosis sly-ly skirts the issue by omitting any reference to phase.10
References:
1. Amini K, Simko E, Davies JL. Diagnos-tic exercise: Sudden death associated with myocardial contraction band necro-sis in boer goat kids. Vet Pathol. 2011; (48):1212–1215.
2. Cooper BJ, Valentine BA. Muscle and Tendon. In: Maxie MG, ed. Jubb, Ken-nedy & Palmer’s Pathology of Domestic Animals. Vol 1. 6th ed. Elsevier; 2016: 164-249.
3. Helmer C, Hannemann R, Humann-Ziehank E, et al. A case of concurrent molybdenosis, secondary copper, cobalt and selenium deficiency in a small sheep herd in Northern Germany. Animals. 2021;11(7):1864.
4. Kennedy S, Rice DA. Histopathologic and ultrastructural myocardial alterations in calves deficient in vitamin E and sele-nium and fed polyunsaturated fatty ac-ids. Vet Pathol. 1992 Mar;29(2):129-38.
5. Miller MA, Lyle T, Zachary JF. Mecha-nisms and Morphology of Cellular Inju-ry, Adaptation, and Death. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th ed. Elsevier; 2022: 47-48.
6. Oakes, SA. Cell Injury, Cell Death, and Adaptations. In: Robbins & Cotran Pathologic Basis of Disease. 10th ed. Elsevier; 2022:65-66.
7. Robinson WF, Robinson NA. Cardio-vascular System. In: Maxie MG, ed. Jubb, Kennedy & Palmer’s Pathology of Domestic Animals. Vol 3. 6th ed. Else-vier; 2016: 1-101.
8. Surai PF, Kochish II, Fisinin VI, Juniper DT. Revisiting oxidative stress and the use of organic selenium in dairy cow nu-trition. Animals (Basel). 2019 Jul;9(7): 462.
9. Valentine BA. Skeletal Muscle. In: Zachary JF, ed. Pathologic Basis of Vet-erinary Disease. 7th ed. Elsevier; 2022:1009, 1018, 1025-1027.