Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 15
22 January 2020
Dr.
Cory Brayton, DVM, PhD, DACVP, DACLAM,
Director, Phenotypic Core
Associate Professor of Molecular and Comparative and Pathobiology
Johns Hopkins School of Medicine
733 N. Broadway
Baltimore MD, 21205
CASE IV: 17N076 (JPC 4103914).
Signalment: ~6 months; Female; NOD.Cg-Prkdcscid IL2rgtm1Wjl/SzJ (aka NOD-scid-gamma, NOD-scid IL-2Rγnull, NSGTM mouse); Mus musculus
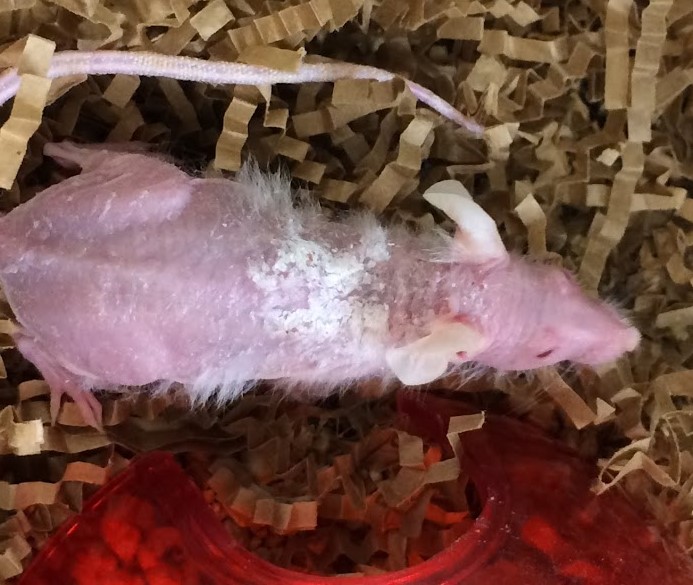
History: A NOD-scid-gamma (NSGTM) mouse was presented for progressive diffuse hair loss and scaling dermatitis. (NSGTM mice are typically fully haired). Four months prior to presentation, the mouse had undergone chemical depletion of the native hematopoietic compartment and adoptive transfer of human CD3+-depleted bone marrow mononuclear cells, followed 2 weeks later by human CD3+ T cell transfer. The experimental intent was to create a humanized hematopoietic system, which could then be used to assess effects of experimental agents on the hematopoietic system.
Gross Pathology: The mouse had complete to partial alopecia affecting the entire hair coat, with scaling dermatitis predominantly on the interscapular dorsum
Laboratory results:
· PCR was performed on skin using primers specific for Corynebacterum bovis: results were negative
· Aerobic culture of skin grew few Staphylococcus aureus and rare Enterococcus faecium
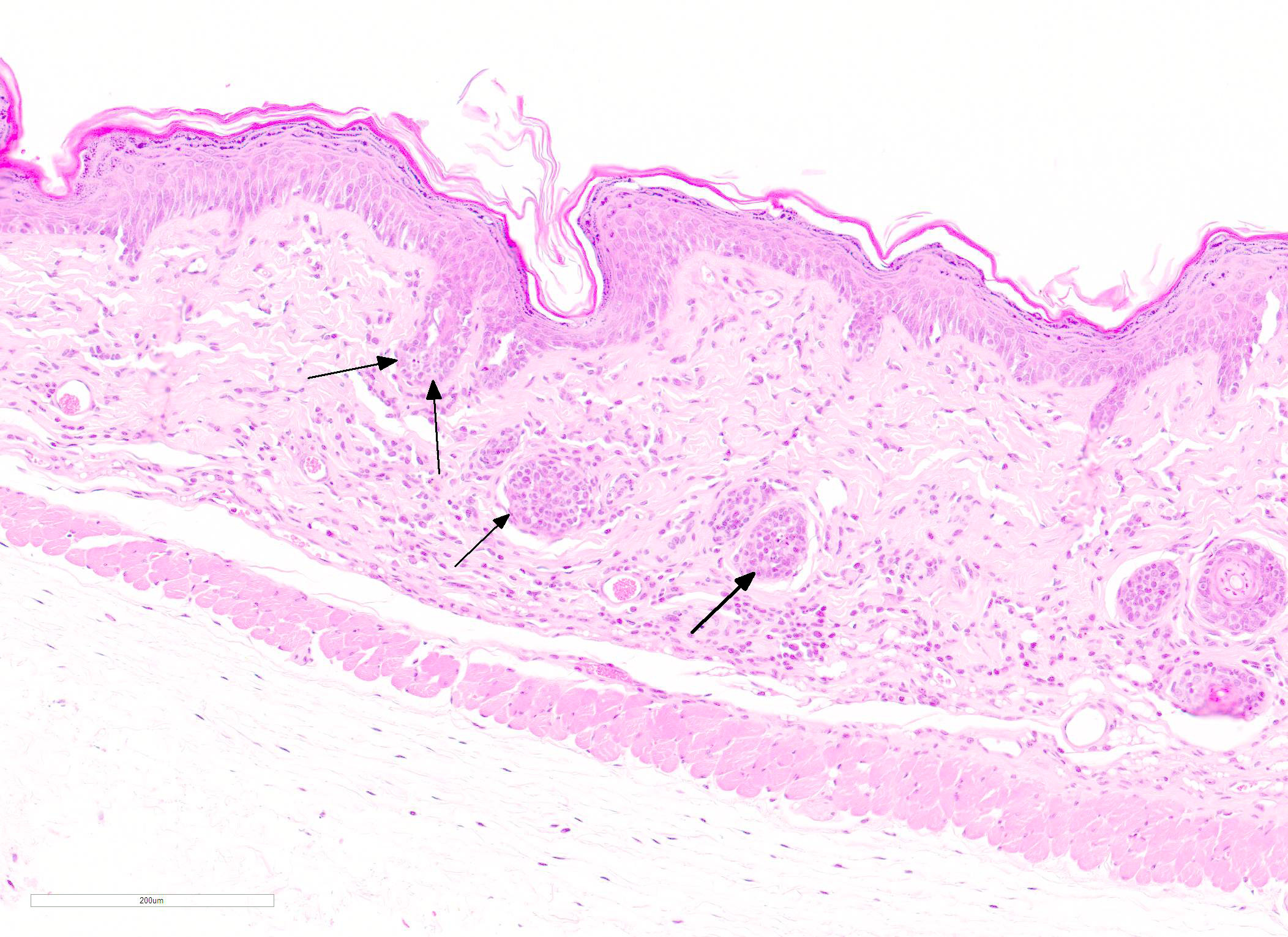
Microscopic Description: Skin: In sections of haired skin, there is multifocal vacuolar change within the basilar epidermal and adnexal epithelium, evidenced by shrinking and separation of adjacent keratinocytes with occasional intracytoplasmic vacuolation and occasional dyskeratosis. Shrunken cells with pyknotic nuclei, consistent with apoptotic keratinocytes, are multifocally present in the same areas. Additionally, there is a mild to moderate interface and adnexal dermatitis comprised of lymphocytes and macrophages adjacent to and infiltrating the basal epithelium, outer root sheath of follicular epithelium, and sebaceous gland epithelium. Multifocal lymphocytic and macrophagic dermal infiltration and mild dermal fibrosis are also present. The overlying epidermis has moderate diffuse orthokeratotic hyperkeratosis and the stratum granulosum is prominent (hypergranulosis).
Other organs evaluated (not shown) included the lungs, liver, kidneys, uterus, spleen, mesenteric lymph nodes, pancreas, gastrointestinal tract, and bone marrow. The lungs contained diffuse peribronchial and peribronchiolar lymphocytic infiltration, with occasional intraepithelial infiltrates. The liver and kidneys showed infrequent, perivascular and peribiliary (in liver) mononuclear to lymphocytic infiltration. The bone marrow, spleen, and lymph nodes were highly cellular with appropriate distribution of erythroid and myeloid precursors in marrow and red pulp and with appropriate lymphoid cellularity and organization in the spleen and lymph nodes.
Contributor Morphologic Diagnosis:
Skin: Dermatitis, interface and adnexal, lymphohistiocytic, with basilar vacuolar change, orthokeratotic hyperkeratosis, and dermal fibrosis, chronic, multifocal, moderate
Contributor Comment: The gross and histologic findings in this case were consistent with graft-vs-host disease secondary to a protocol intended to generate immunologically ?humanized? mice.
NSGTM mice2,7 have three immune-related defects, consisting of:
1) The genetic background NOD/ShiLtJ, which is a polygenic defect of the innate immune system resulting in impaired phagocytosis and defective antigen presentation by macrophages and dendritic cells
2) Severe combined immunodeficiency (?scid?) mutation arising from loss of function of the gene Prkdc. Prkdc encodes the catalytic subunit of the protein complex that controls ligation of V(D)J DNA fragments during T cell receptor or immunoglobulin gene recombination in lymphocytes. Its absence results in failure to generate mature, functional B or T lymphocytes.
3) A targeted null mutation of the IL2 receptor γ chain, which blocks receptor recognition of cytokines IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. This further impacts murine hematopoietic development and results in a lack of mature, functional NK cells (development requires IL-15).
Because of these broad immunologic defects, NSGTM mice are often the recipient strain of choice for experimental engraftment, including the transfer of human hematopoietic cells to generate mice with a ?humanized? immune system.2,7 These humanized mouse protocols involve irradiation or chemical depletion of the native murine hematopoietic compartment and transfer of either human fetal bone marrow, liver, and thymus (BLT) or human bone marrow or peripheral blood-derived CD34+ stem cells. Engrafted cells migrate to bone marrow and differentiate to all lineages of the mature immune system.
Humanized mouse protocols carry the potential for development of graft-vs-host disease (GVHD), in which human donor T cells are primed against murine antigens presented by graft-derived human cells, generating an immune response against murine host tissues. To avoid this, grafts are typically depleted of mature CD3+ cells to allow immature T cells, which will ostensibly regard host tissues as ?self?, to develop from the graft. Nevertheless, GVHD has been reported in humanized NSGTM, most commonly in mice receiving BLT, but occasionally in mice receiving CD34+-selected, CD3+-depleted stem cells grafts.2-5 Engraftment of non-CD3+-depleted, peripheral blood mononuclear cells (PBMCs) has also been used to purposefully induce GVHD for study of pathogenesis or interventional strategies.1,3
In this mouse, the most severely affected organs were the skin and the lung. Organs affected by GVHD in NSGTM mice resemble those targeted in human GVHD, with skin, lung, liver, gingiva, and intestinal tract variably affected, depending on the type of graft, the individual mouse, or the stage of disease.3,6 Organ specificity may relate to T cell homing receptor expression.1 GVHD can occur acutely or have a more insidious, chronic course. Chronic GVHD may have a more complicated pathogenesis involving autoantibody production and impaired development of central or peripheral tolerance in graft-derived T cells. In both acute and chronic GVHD, MHC I-mediated presentation of murine antigen by graft-derived cells plays a key role, as GVHD preferentially occurs in mice receiving human grafts with specific HLA class I antigen haplotypes, particularly those associated with autoimmune disease in humans.5 GVHD in this mouse clinically resembled chronic GVHD as it occurred 4 months after grafting- this is much longer than the typical presentation of acute GVHD in NSG mice (3-4 weeks post-engraftment).9
The key feature of cutaneous GVHD is interface dermatitis with basilar keratinocyte apoptosis.5 This ranges from a mild interface dermatitis with vacuolar change to a lichenoid (band-like) infiltration. In chronic GVHD, orthokeratotic hyperkeratosis and dermal fibrosis (dermal sclerosis) become more prominent and inflammation may be more severe, although there is considerable variation and features of acute and chronic GVHD may be present in the same individual.5
The key feature of chronic pulmonary GVHD in humans is the appearance of bronchiolitis obliterans, believed to result from chronic inflammation and fibrotic remodeling of small airways. In this mouse case, bronchiolitis obliterans was not apparent and changes were confined to a pronounced lymphohistiocytic bronchiolitis. It may be that fibrotic changes of the airway would develop with time. A recent survey of human patients with clinically suspected GVHD-associated bronchiolitis obliterans showed that 9 of 33 biopsies showed only lymphocytic bronchiolitis without fibrosis ?this may represent an earlier stage of disease progression.5
Contributing Institution:
Unit for Laboratory Animal Medicine
In Vivo Animal Core
University of Michigan
2800 Plymouth Road, B36-G178
Ann Arbor, MI 48109
http://animalcare.umich.edu/business-services/vivo-animal-core
JPC Diagnosis: Haired skin: Dermatitis, lymphohistiocytic, diffuse, mild to moderate, with epidermal and follicular basal cell apoptosis, intra- and extracellular edema, epidermal hyperplasia, hypergranulosis, and orthokeratotic hyperkeratosis.
JPC Comment: For a wide range of hematologic malignancies, a cure is only availble through the use of allogeneic hematologic stem cell transplants (allo-HSCT).4,7,8 Over a million of these procedures have been completed within the last decade4, in which a combination of radation and chemotherapy is used to eliminate neoplastic or genetically abnormal cells of the hematopoetic compartment which are then replaced with hematopoetic stem cells from an allogeneic donor possessing differences in human leucocyte antigens (HLA) or major (MHA) and minor histocompatibility antigens (miH).4 In the most simple terms, the syndrome of graft-versus host disease results from when cells from an immunocompetent donor recognize and attack the tissues of the immunocompromised recipient (who is incapable of mounting a response against the donor?s cells). GHVD is a major cause of morbidity in allo-HSCT recipients, affecting up to 40-60%, and also accounting for 15% of mortality.7
In humans, cutaneous GVHD is considered the earliest and most common manifestation and may wraant a poor progosis.4,7 A macular rash affecting a number of body parts, including the palms and soles, is its hallmark. This condition, in addition to vomiting and jaundice resulting from small bile duct damage and cholestasis, forms the basis for early diagnosis of acute GVHD.8
GVHD is divided into classic acute GVHD in which symptoms appear within 100 days (often 2-3 weeks following engraftment) and are consistent with the triad described above, and late onset acute GVHD which also shares these symptoms but appears after 100 days. Chronic GVHD represents 50% of all cases and 25% of mortalities occurs after the 100 day period.
In humans, the rash seen in aGVHD are usually centered on hair follicles, a useful early sign for diagnosis.4,7 Histologically, the lesion in humans is very similar to that seen in the mouse model (and in this case), with vacuolar degeneration of the basal layer, dyskeratotic keratinocytes, and a mild lymphocytic infitrate at the dermo-epidermal junction that may infiltrate the epidermis beginning at the rete ridges and hair follicles.7 The population of lymphocytes is predominantly CD4+ and CD8+, and their presence in the epidermis suggests that they are donor lymphocytes attacking keratinocytes expressing different MHA antigens.8
Chronic forms of cutaneous GVHD (cGVDH) appear to be more common overall, and are reported in between 60 and 80% of patients. The most commonly recognized forms include lichenoid GVHD and sclerodermatous cGVHD. Lichenoid cGVHD exhibits most of the histologic signs descrbed about, with a macular hyperkeratotic rash. Sclerodermatous cGVHD may result in fibrosis at any level of the skin and ultimately may be disfiguring.7
One positive aspect of GVHD is associated with an effect known as graft-versus-leukemia (GVL). It well-documented that these patients have lower relapse rates of hematologic malignancies, and is a useful benefit in paitents with reduced intesity regimens to clear the body of neoplastic hematologic cells.4
Many participants had Corynebacterium bovis on ther list of differentials, but the moderator pointed out the lack of follicular hyperkeratosis, the mininal inflammation, and the proliferation of the stratum spongiosum, rather than the stratum granulosum, as in this case.
References:
1. Ali N, Flutter B, Sanchez Rodriguez R, et al. Xenogeneic graft-vs-host disease in NOD-scid IL-2Rγnull mice display a T-effector memory phenotype. PLoS One. 2012; 7(8):e44219.
2. Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor (gamma) chain (null) mice. Blood. 2005; 106(5):1565-73.PMC1895228.
3. King MA, Covassin L, Brehm MA, et al. Human peripheral blood leukocyte non-obese diabetic-severe combined immunodeficiency-interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-vs-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009;157(1):104-18.
4. Rodriguez KS, Oliverira-Ribeiro C, de Abreu Fiuza Gomes S, Knobler R. Am J Clin Dermatol 2018; 19:33-50.
5. Shulman HM, Cardona DM, Greenson JK, et al. Histopathologic diagnosis of chronic graft-vs-host disease. NIH consensus development project on criteria for clinical trials in chronic graft-vs-host disease. II. The 2014 Pathology Working group report. Biol Blood Marrow Transplant. 2014; 21(4):589-603.
6. Sonntag K, Eckert F, Welker C, et al. Chronic graft-vs-host-disease in CD34+ humanized NSGTM mice is associated with human susceptibility HLA haplotypes for autoimmune disease. J Autoimmun. 2015; 62:55-66.
7. Villareal CDV, Perez JCJ, Alanis JCS, Candiani JC. Cutaneous graft-versus-host disease after hematopoietic stem cell transplant ? a review. An Bras Dermatol 2016; 91(3):336-343.
8. Zeiser R, Blazar BR. Acite graft-versus-Host diseas biology, prevention, and therapy.
9. https://www.jax.org/strain/005557