Signalment:
Gross Description:
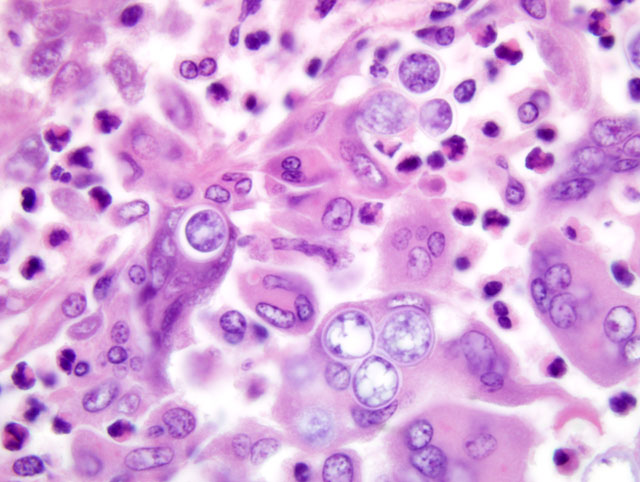
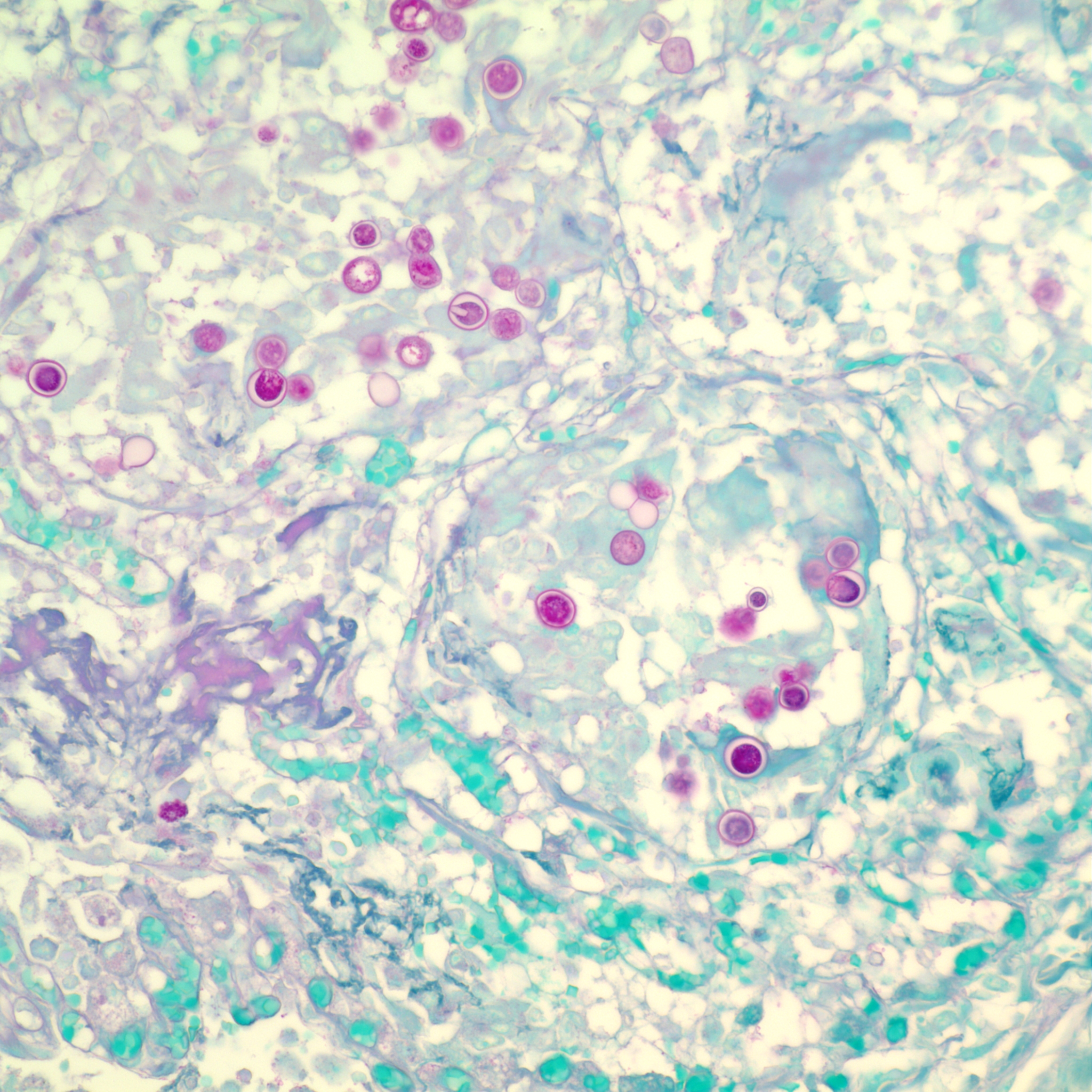
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Geographically, the disease mainly occurs in North America, with occasional infections in Africa, Europe, Asia and Central America. In North America, the Mississippi, Ohio and St. Lawrence river basins; northern Ontario; the Mid- Atlantic states; and the Canadian provinces of Quebec and Manitoba have had endemic infections.(1) Sporadic cases have been described in New York. The prevalence of blastomycosis is 10 times higher in dogs than in humans, with occasional simultaneous infections; therefore, dogs are considered sentinels for human disease.(2)
Blastomyces dermatitidis is thought to originate from the soil; however, the organism is not commonly recovered at the suspected exposure sites. In the environment it grows as mycelia, requiring sandy, acidic soil and proximity to water; it causes disease to animals by inhalation of the spores.(1) The mycelial form converts to the yeast form in the terminal bronchioles of the lung, then disseminates to other preferred sites in the body via the blood and lymphatic vessels. In dogs these include the skin, eyes, bones, lymph nodes, subcutaneous tissues, external nares, brain and testes. Less commonly the mouth, nasal passages, prostate, liver, mammary gland, vulva and heart also might be affected. Skin lesions may be caused by local inoculation. Intestinal lesions are uncommon in dogs with systemic disease. Subclinically infected dogs are very rare. Experimentally infected dogs develop mild lesions, but with much higher frequency than for other systemic mycoses. However, the disease in experimentally infected dogs is mostly mild and the animals recover without treatment.(4) Lung lesions may resolve by the time other organs present with signs of infection. The lesions normally present as either granulomas with many epithelioid and giant cells, or pyogranulomatous foci that consist of necrotic neutrophils and macrophages.(6)
The virulence factors of B. dermatitidis remain mostly unknown and naturally infected animals may have a suppressed immune response, the mechanism of which is not clear. Moreover, it has not yet been determined whether dissemination of the disease depends upon a compromised immune system.(1) An effective immune response requires T lymphocytes targeting a surface adhesion virulence factor Blastomyces adhesion 1 (BAD-1) immunodominant antigen (formerly called Wisconsin 1 (WI-1)), a molecule that mediates attachment to the host cells and blocks tumor necrosis factor (TNF)-α production. Antibody responses to BAD-1, although associated with reduced disease severity, were not able to entirely protect against the infection. Moreover, the cell wall polysaccharide α-glucan was also shown to be associated with virulence and to inhibit elimination by macrophages. (6)
Cytologic or histologic evaluation can be used to identify the organism. Usually, the diagnosis is made by demonstrating the yeast bodies in tissue sections or cytologic preparations. Safety measures must be taken because laboratory personnel can become infected by the cultured mycelia.(1,6) Serologic tests are also available but commonly produce false negative results. Measurement of Blastomyces antigen in urine or serum by antigen enzyme immunoassay (EIA) is more sensitive than measurement of anti-Blastomyces antibodies for the diagnosis of blastomycosis in dogs.(8)
Hypercalcemia has been associated with blastomycosis, and is likely caused by abnormal vitamin D metabolism. The kidneys provide the site of hydroxylation of 25-hydroxy-cholecalciferol to produce active 1,25- dehydroxycholecalciferol. Studies suggest, however, that granulomas composed primarily of monocyte derived phagocytes can metabolize 25-hydroxyl-cholecalciferol to calcitriol in vitro, providing the extrarenal source for hypercalcemia associated with blastomycosis. The production of calcitriol by the granulomas seems to be de-regulated or mediated differently than by the kidneys.(3)
Multinodular lesions in the lung and other organs must be differentiated from those of other systemic mycoses and metastatic neoplasia that can be distinguished by examination of impression smears or histologic sections. Neoplastic nodules tend to be larger and more variable in size. Other fungi are normally differentiated from Blastomyces based on morphologic features. Blastomyces cells are yeast-like, multinucleated, spherical, 8-20 μm in diameter, possess thick double walls, and produce single, broad-based buds. Histoplasma capsulatum is much smaller (2-4 μm) and found in the cytoplasm of macrophages. Cryptococcus neoformans has a thick capsule and cause a mild inflammatory response. Mutant forms of Cyptococcus lacking the typical thick capsule might resemble Blastomyces and induce a similar granulomatous response, but this fungus displays narrow-based budding. Coccidioides immitis is much larger (20-200 μm) and contains endospores. Blastomyces, Cryptococcus and Histoplasma can be differentiated in culture by the presence and morphology of mycelia, conidia, and yeast, respectively.(1) Histoplasma capsulatum var. duboisii (African histoplasmosis) has narrow-based buds and is not multinucleated. Aspergillus organisms are septate, thin, and parallel walled with acute-angle dichotomous branching. Paracoccidioides braziliensis causes South American blastomycosis and reproduces in tissues by multiple budding.(4)
JPC Diagnosis:
Conference Comment:
Careful consideration of the molecular basis for histomorphologic changes is often useful in their proper interpretation. Both the TH1 and TH2 responses are important in cell-mediated immunity to invasive fungi; however, they result in dissimilar histomorphologic changes. The former is typified by the formation of discrete granulomas with the hallmark being epithelioid macrophages, while in the latter neutrophils and activated monocytes and macrophages predominate.(5,7) Conference participants reviewed the mechanisms responsible for TH1 and TH17 responses in detail.
In response to intracellular bacteria and fungi, dendritic cells present fungal antigen to na+�-�ve T cells and secrete interleukin (IL)-12 which, along with interferon (IFN)-γ, initiates the differentiation of na+�-�ve T cells into TH1 cells. This differentiation requires the lineage-specific transcription factor T-bet; differentiated TH1 cells characteristically produce IFN-γ and IL-12. Interferon-γ further amplifies the differentiation of TH1 cells in an autocrine loop, and inhibits the differentiation of TH17 cells, potent recruiters of neutrophils and monocytes involved in the host defense against extracellular bacteria and fungi. Interferon-γ induces isotype switching in B cells to favor IgG production, and causes a number of functional and morphological alterations in macrophages, resulting in their activation, e.g. enhanced phagocytosis and killing ability; increased class II major histocompatibility complex expression; and increased production of TNF-α, IL-12, and other proinflammatory cytokines. Interleukin-12, acting synergistically with IL-18, further amplifies the TH1 response. Tumor necrosis factor-α is an important cytokine in phagocytemediated killing of yeast; thus, as mentioned by the contributor, its depressed production in the presence of the BAD-1 antigen impairs the cell-mediated immunity that is vital to the host defense against Blastomyces dermatitidis. (5,7)
In response to extracellular bacteria and fungi, na+�-�ve T cells under the control of transforming growth factor (TGF)- β plus IL-6 and IL-1, or TGF-β plus IL-21 followed by IL-23, undergo differentiation into TH17 cells. Transforming growth factor-β, classically characterized as an immunosuppressive cytokine, induces the expression of forkhead box P3 (Foxp3) in na+�-�ve T cells, driving the induction of regulatory T cells that suppress inflammation. Interleukin-6 is a potent inhibitor of this pathway, and in combination with TGF-β and IL-1, drives the expression of IL-17 by na+�-�ve T cells, committing them to the TH17 lineage. Activation of the transcription factor ROR-γt in cells committed to the TH17 lineage causes expression of the receptor for IL-23. Interleukin-23 exposure to these developing TH17 cells enhances IL-17 expression, induces IL-22 expression, and suppresses the expression of IL-10 and IFN-γ, thus stabilizing the TH17 phenotype. Unlike the signature cytokines produced by cells of the TH1 and TH2 pathways (i.e. IFN-γ and IL-4, respectively), IL-17 produced by TH17 cells does not amplify TH17 responses in an autocrine loop; rather, IL-21, produced by mature TH17 cells, in combination with TGF-β, amplifies TH17 differentiation. Notably, the differentiation of TH17 cells is inhibited by IFN-γ (TH1 pathway) and IL-4 (TH2 pathway).(5,7)
References:
2. Cote E, Barr SC, Allen C, Eaglefeather E: Blastomycosis in six dogs in New York state. J Am Vet Med Assoc 210:502-504, 1997
3. Dow SW, Legendre AM, Stiff M, Greene C: Hypercalcemia associated with blastomycosis in dogs. J Am Vet Med Assoc 188:706-709, 1986
4. Jones TC, Hunt RD, King NW: Veterinary Pathology, 6th ed., pp. 508-510. Williams and Wilkins, Baltimore, MD, 1997
5. Kumar V, Abbas AK, Fausto N, Aster JC: Diseases of the immune system. In: Robbins and Cotran Pathologic Basis of Disease, eds. Kumar V, Abbas AK, Fausto N, Aster JC, 8th ed., pp. 183-201. Saunders Elsevier, Philadelphia, PA, 2010
6. Legendre A: Blastomycosis. In: Infectious Diseases of the Dog and Cat, ed. Greene CE, 3rd ed., pp. 569-576. Saunders, St. Louis, MO, 2006
7. Miossec P, Korn T, Kuchroo VK: Interleukin-17 and type 17 helper T cells. N Engl J Med 361:888-898, 2009
8. Spector D, Legendre AM, Wheat J, Bemis D, Rohrbach B, Taboada J, Durkin M: Antigen and antibody testing for the diagnosis of blastomycosis in dogs. J Vet Intern Med 22:839-843, 2008
9. Wilkinson LM, Wallace JM, Cline JM: Disseminated blastomycosis in a rhesus monkey (Macaca mulatta). Vet Pathol 36:460-462, 1997