Wednesday Slide Conference, 2025-2026, Conference 6, Case 3
Signalment:
9 year 7 months old, male neutered, domestic short haired cat, Felis Cattus.History:
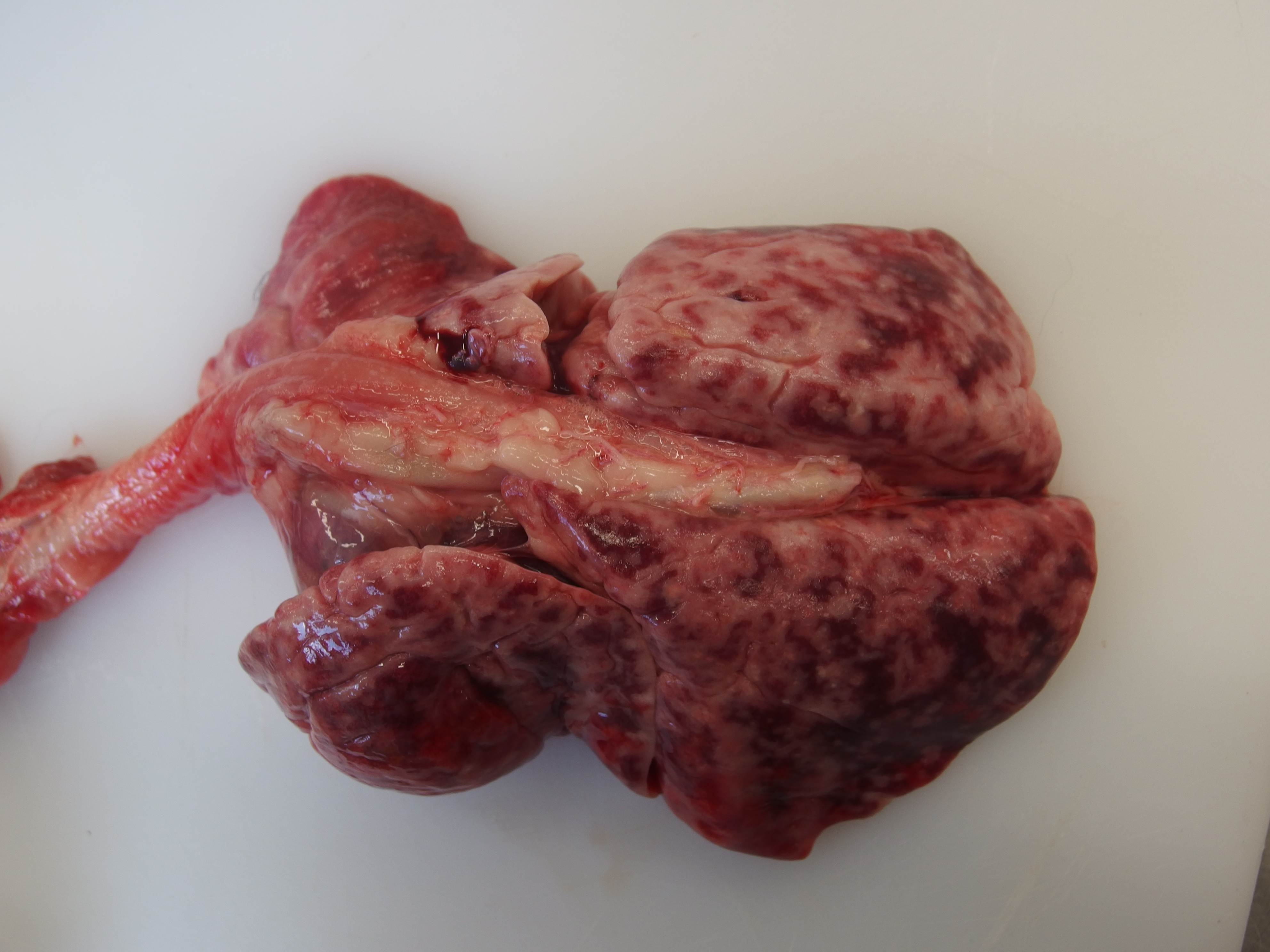
Gross Pathology: Diffusely all lung lobes were markedly expanded by moderately firm red-cream tissue. The pleural surfaces were smooth despite the lungs being expanded.The pericardial sac contained approximately 2mL of serosanguinous fluid. The heart weighed 28 g (cardiomegaly, <20g is considered normal). Cross section of the left ventricular free wall, interventricular septum, and right ventricular free wall measured 6 mm, 6 mm, 3 mm respectively (cardiac hypertrophy with right ventricular free wall most prominently affected). This results in a ratio between the chamber widths of 2:2:1. The left and right auricles were approximately 15 mm in length and width, and 5-10 mm in depth, and were both similar sizes (bilateral auricular dilation).
Laboratory Results:
Anemia and thrombocytopenia diagnosed on hematology at first presentation in March to the referring vet practice.Microscopic Description:
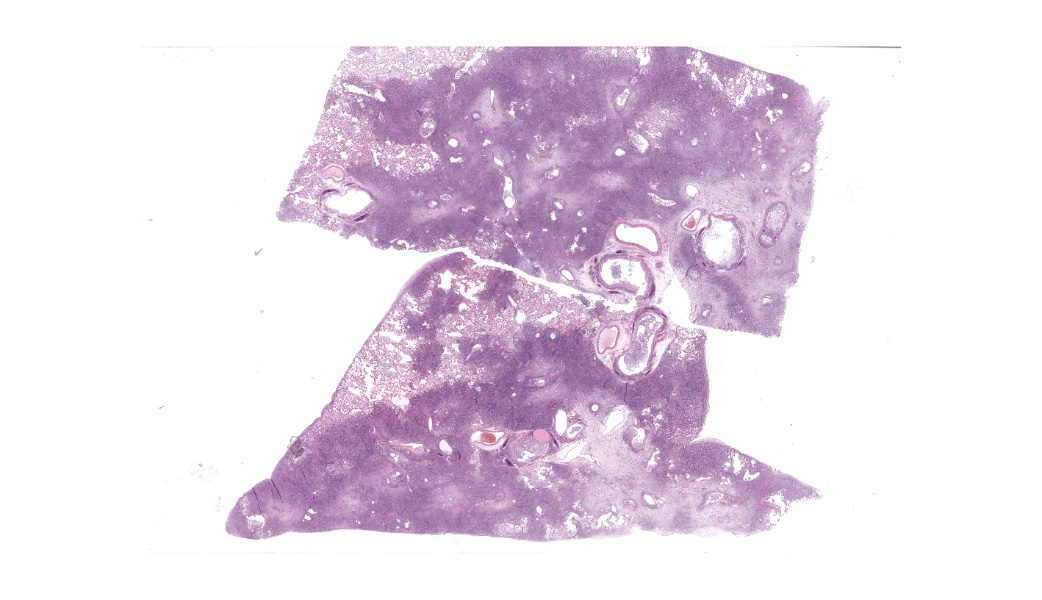
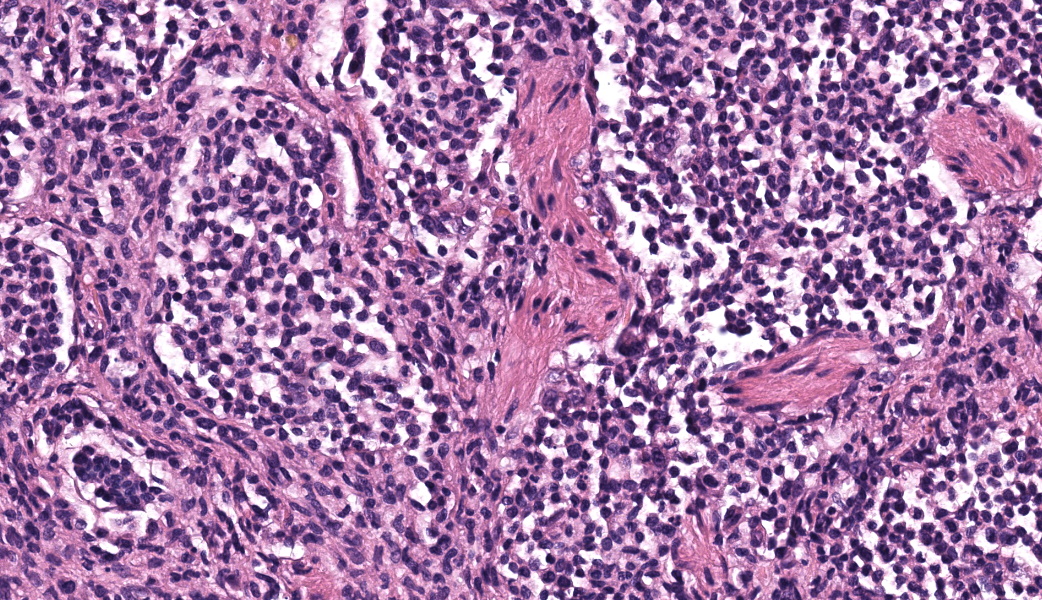
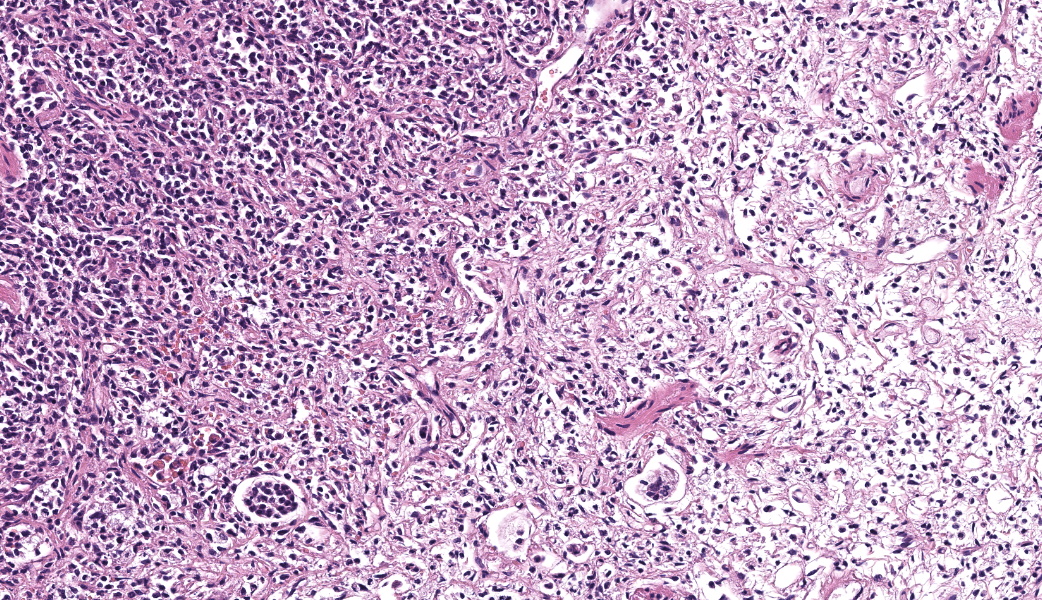
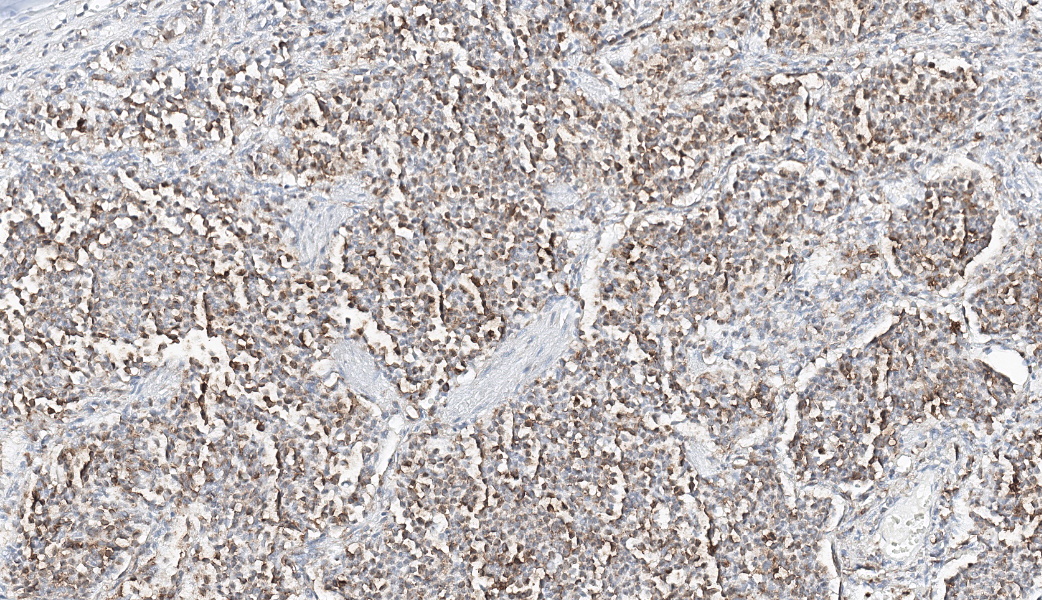
Expanding the airway and alveolar interstitium, subpleural spaces and also filling alveolar spaces, there is a proliferation of large numbers of round-polygonal cells. The proliferative round cell population have a moderate amount of eosinophilic cytoplasm and a reniform nucleus that contains coarsely stippled chromatin and a single nucleolus. There is mild anisocytosis and anisokaryosis, and there are occasional multinucleated cells. There is one mitotic figure per 2.37 square mm. Within the pleural, peribronchiolar interstitium and alveolar septa, the proliferative cells are accompanied by fine strands of collagen, resulting in further expansion of those structures, and in some areas, complete effacement of the normal pulmonary architecture with obliteration of air spaces. In non-consolidated areas, alveolar septa are multifocally ruptured (microscopic emphysema). There is multifocal, moderate, hyperplasia of bronchiolar smooth muscle, and occasionally, especially in subpleural areas, there is type 2 pneumocyte hyperplasia. Medium-sized pulmonary arteries demonstrate hyperplasia of the tunica media. Airway epithelium is frequently sloughed (post-mortem artefact). Gram, Ziehl-Neelson and PAS-stained sections do not highlight micro-organisms.Contributor's Morphologic Diagnoses:
Lung, feline pulmonary histiocytosisEtiological diagnosis: Feline pulmonary Langerhans cells histiocytosis
Contributor's Comment:
Feline pulmonary Langerhans cell histiocytosis is a proliferative disorder of Langerhans cells predominantly affecting the lung. The process is progressive, with animals ultimately developing fatal respiratory compromise. The condition has been reported to have multi-organ involvement and may affect the spleen, as in this case.3Initial clinical signs of feline pulmonary Langerhans cell histiocytosis are variable but ultimately progress to a restrictive dyspnoea.1 The insidious onset and unspecific clinical signs means that definitive diagnosis in live patients is challenging. Langerhans cells express CD1a, MHC-class 2, langerin (CD207) and E-cadherin.6,8
In humans, the condition is associated with young adults who smoke. The exact pathogenesis of the condition is uncertain.3 Current evidence from human medicine suggests the underlying process is a myeloid neoplasm with inflammatory properties, which may regress or progress in response to cessation of smoking.13 In cats, reactivity or neoplastic has not been defined.
The condition generally affects middle aged to older cats, with an interstitial and alveolar pattern on lung imaging.1 Similar to the eccentric cardiac hypertrophy in this case, occasionally cats with Feline progressive pulmonary histiocytosis develop right ventricular hypertrophy and right auricle dilation due to the increase in pulmonary pressure.14
Grossly, lungs have an expanded non-collapsing texture and contain multifocal pale red-cream coalescing masses, which can be very extensive in distribution. In advanced cases the entire lung lobes can be affected, with extension of masses into the pleura.12 The pancreas, kidney, liver and local or remote lymph nodes may also contain similar mass lesions.2
Typical microscopic findings include pleomorphic histiocytic cells that target terminal and respiratory bronchioles and extend into adjacent alveoli.3 Affected bronchioles undergo smooth muscle hyperplasia and there is fibrosis of the alveolar septa. The Langerhans cells demonstrate CD18 and E-cadherin, vimentin and IBA-1positive immunoreactivity.1 In addition, feline Langerhans cells do not react to CD204.7
The presence of Birbeck granules in the cytoplasm ultrastructurally is characteristic of the Langhans cell phenotype in cats.3,4 Birbeck granules are the langerin (CD207) receptor cross-linked to antibody and subsequently internalised from the cell surface membrane.9 Canine Langerhans cells do not contain Birbeck granules.12
Feline pulmonary Langerhans cell histiocytosis has been diagnosed on the basis of BAL cytology and pulmonary radiographs.9 Thoracic radiographs have miliary to nodular radiodense foci in a bronchointerstitial pattern.10
Other progressive histiocytic diseases that affect the lungs include progressive histiocytosis in cats, and cutaneous Langerhans histiocytosis or systemic histiocytosis in dogs.10 The microscopic appearance between canine and feline Langerhans histiocytosis in the lungs is similar, with a primary focus on terminal bronchioles.12 Feline progressive pulmonary histiocytosis has been reported in a lion,14 and focal Langerhans cell histiocytosis, without lung involvement, has been reported in the pancreas of a cat.11
Contributing Institution:
University of Cambridge, Madingley Road, Cambridge UKJPC Diagnoses:
Lung: Histiocytosis, alveolar, chronic, diffuse, severe, with fibrosis and smooth muscle hyperplasia.JPC Comment:
The contributor does an excellent job of summarizing this condition in cats and cross-comparing with a few other species affected by a similar process (including humans). Many of the topics mentioned in their write-up were focal points of discussion during conference, including key histologic features, IHC immunoreactivity profile, and ultrastructural findings.Langerhans cells are antigen-presenting dendritic cells (DC) derived from CD34+ precursors in the bone marrow. They eventually make their way to and reside in the epidermis and epithelium of the oral cavity, vagina, and bronchial mucosa of the lung.3 Upon contacting and internalizing an antigen, Langerhans cells will migrate to regional lymph nodes and present their cool find (the antigen) to naïve CD4+ T-cells using major histocompatibility complex (MHC) class II. The characteristic “Birbeck’s granules” seen on electron microscopy (EM) are five-layered, rod-shaped cytoplasmic organelles that kind of resemble hot dogs on a bun and are present only within Langerhans cells. These granules are formed by the internalization of CD207, normally found on the surface of Langerhans cell. CD207 is a C-type lectin also known as “langerin” that assists with recognition of pathogen-associated molecular patterns (PAMPs), internalization of an antigen, and further stimulating an immune response as described above.3
While the pathogenesis of feline pulmonary Langerhans cell histiocytosis is not yet fully understood, in humans, one of the main hypotheses for pulmonary Langerhans cell histiocytosis is that of the “cytokine storm”.3,5 This hypothesis suggests that T-lymphocytes and Langerhans cells within the lungs produce a mutually amplifying cytokine cascade that drives recruitment, maturation, and proliferation of Langerhans cells in this condition.5 Whether or not this fits within a reactive or neoplastic process remains to be seen, and some studies suggest assessing the clonality of the Langerhans cells in future research to try and make that distinction.3
References:
- Argenta FF, de Britto FC, Pereira PR, et al. Pulmonary Langerhans cell histiocytosis in cats and a literature review of feline histiocytic diseases. J Feline Med Surg. 2020;22(4):305-312.
- Bellamy E, Di Palma S, Ressel L, et al. Disseminated Langerhans cell histiocytosis presenting as oesophageal disease in a cat. JFMS Open Rep. 2019;5(2):2055116919874902.
- Busch MD, Reilly CM, Luff JA, et al. Feline pulmonary Langerhans cell histiocytosis with multiorgan involvement. Vet Pathol. 2008;45(6):816-24.
- Caswell JL, Williams KJ. Respiratory System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier Ltd. 2016:499.
- Egeler RM, Favara BE, van Meurs M, Laman JD, Claassen E. Differential In situ cytokine profiles of Langerhans-like cells and T cells in Langerhans cell histiocytosis: abundant expression of cytokines relevant to disease and treatment. Blood. 1999;94(12):4195-201.
- Fulmer AK, Mauldin GE. Canine histiocytic neoplasia: an overview. Can Vet J. 2007;48(10):1041-3,1046-50.
- Hirabayashi M, Chambers JK, Sumi A, et al. Immunophenotyping of nonneoplastic and neoplastic histiocytes in cats and characterization of a novel cell line derived from feline progressive histiocytosis. Vet Pathol. 2020;57(6):758-773.
- Laman JD, Leenen PJ, Annels NE, et al. Langerhans-cell histiocytosis 'insight into DC biology'. Trends Immunol. 2003;24(4):190-6.
- Mau A, Chiu ES, Armien A, et al. Antemortem cytologic diagnosis of pulmonary Langerhans cell histiocytosis in a cat. Vet Clin Pathol. 2023;52(4):691-697.
- Moore PF. A review of histiocytic diseases of dogs and cats. Vet Pathol. 2014;51(1):167-84.
- Rissi DR, Brown CA, Gendron K, et al. Pancreatic Langerhans cell histiocytosis in a cat. J Vet Diagn Invest. 2019;31(6):859-863.
- Valli VEO, Kiupel M, Bienzle D. Hematopoietic System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 3. 6th ed. Philadelphia, PA: Elsevier Ltd. 2016 245-247.
- Vassallo R, Harari S, Tazi A Current understanding and management of pulmonary Langerhans cell histiocytosis Thorax 2017;72:937-945.
- Zhang L, Chen H, Ding Y, et al. Pulmonary Langerhans Cell Histiocytosis in an African Lion: A Rare Case Report. Animals (Basel). 2024;14(7):1011.