Signalment:
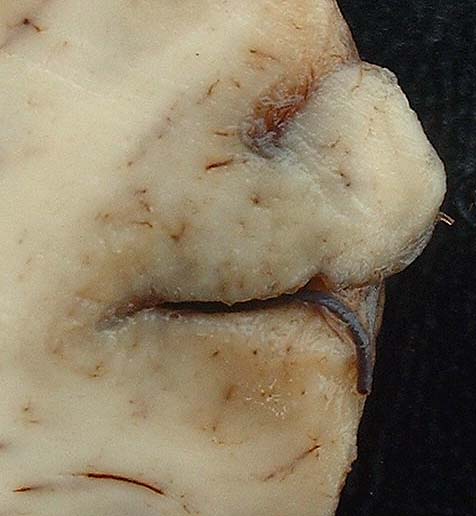
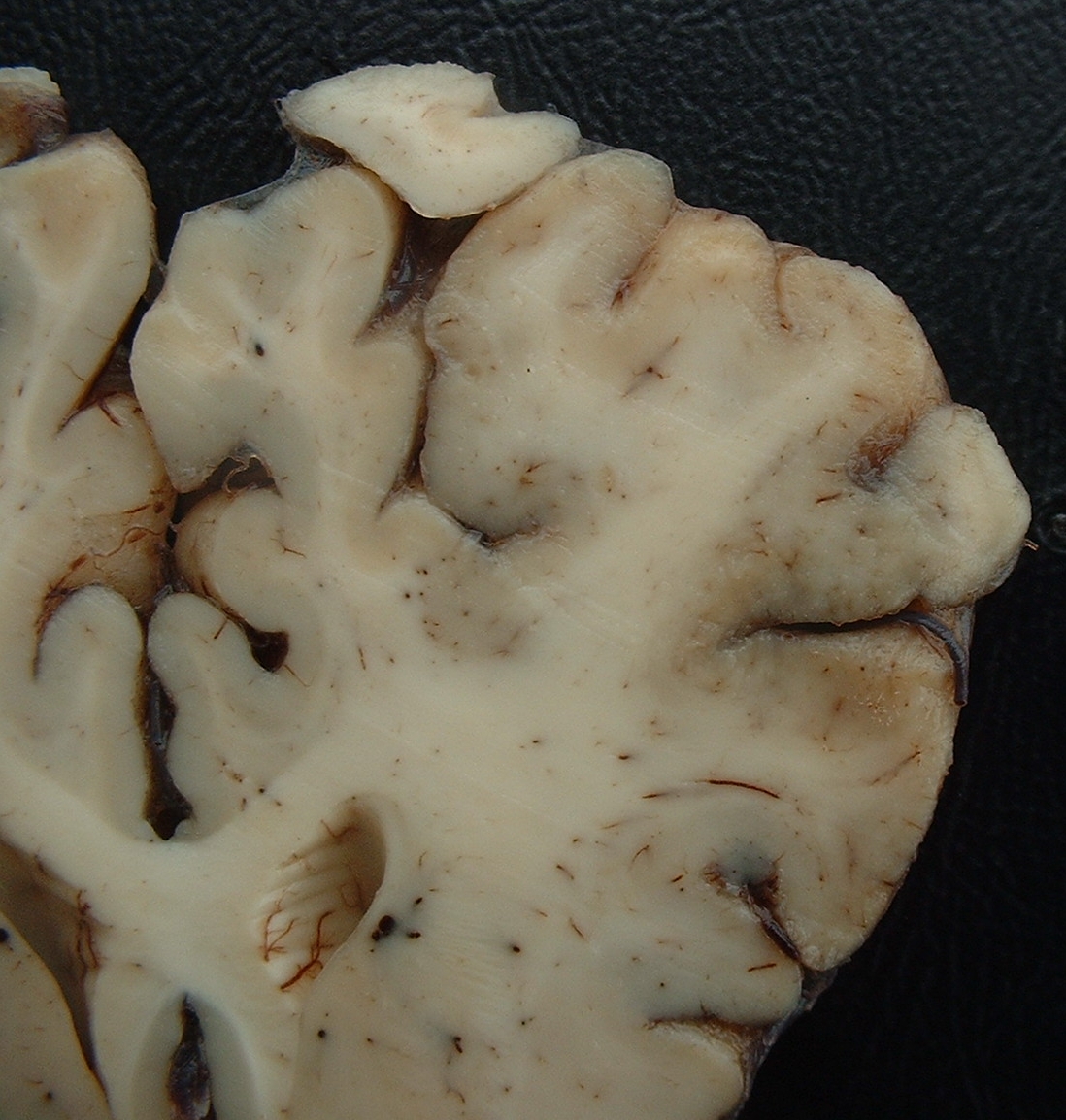
Gross Description:
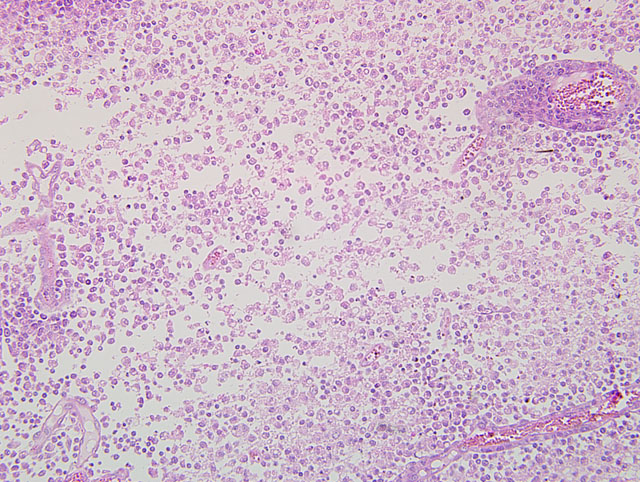
Histopathologic Description:
Morphologic Diagnosis:
1. Brain, cerebral cortex: Malacia, focally extensive, and encephalitis, subacute, accentuated with intranuclear inclusion bodies in astrocytes and neurons, etiology consistent with Bovine herpesvirus type-5, red Angus, calf.
2. Brain,meninges:Meningitis,diffuse,moderate.
Condition:
Contributor Comment:
After acute infection, viruses of the alphaherpesvirus family establish latency in neurons of the sensorial ganglia. Experimental studies demonstrated that the latent infection can be reactivated by the administration of synthetic corticosteroids.6 In natural outbreaks of the disease it is reported that stress, such as weaning, transportation, vaccination, confinement, and sudden variations in temperature can reactivate latent BoHV-5 infection. Rabbits and sheep had been used as models for acute disease and latency.(8) Several pathways have been proposed for the invasion of BoHV-5 in the CNS. It can occur by hematogenous route, because cattle experimentally infected present a phase of viremia. The invasion can occur also through the nervous fibers of the respiratory mucosa. Neuronal dissemination occurs by retrograde, intra-axonal transport in local neurons.(4) Experimental infections in calves5 and rabbits1 suggest that viral infection of the brain occurs more directly along olfactory nerves.
Necrotizing meningoencephalitis caused by BoHV-5 is frequent in Brazil and Argentina, affecting mainly cattle seven months to two years of age. Calves (14 days to 3 months) and adult cattle (up to 4-5 years) are occasionally affected.(2,3,7) Morbidity is variable; in calves it can be up to 30%, with a fatality rate of nearly 100%. In cattle after weaning morbidity is 3-8%. In most outbreaks the fatality rate is 100%, but in some instances a fatality rate of 50-75% has been reported. Sporadic cases also occur in cattle of different ages. Cattle up to 6 years old can be affected. Clinical signs are blindness, deep depression, and other signs of cerebral involvement. Opisthotonos and signs of brain stem lesions, including tongue paralysis, nystagmus, ataxia, other gait alterations, and excessive salivation are reported. Anorexia and severe weight loss can also occur. The clinical manifestation period is 1-15 days.
One common fact in cases of BoHV-5 encephalitis in Brazil is the presence of deep lesions of malacia in basal nuclei, thalamus, and mesencephalon similar to those reported in polioencephalomalacia (PEM). In Brazil, outbreaks of meningoencephalitis by BoHV-5 and outbreaks of PEM show similar epidemiological features, suggesting that meningoencephalitis may be associated with reactivation of a latent BoHV-5 infection during the development of PEM.2 Both diseases affect grazing cattle bred extensively, and do not show any particular seasonal distribution. In addition, the age of affected cattle is similar. The hypothesis that BoHV-5 can be reactivated in cattle which develop PEM, and this reactivation results in severe encephalitis and PEM, was demonstrated, at least experimentally, in cattle infected experimentally by BoHV-5 that received ammonium sulphate from days 114 to 180 after inoculation. One out of 3 cattle developed BoHV-5 meningoencephalitis with lesions of PEM. Other animals recovered after the development of clinical signs but continued to manifest chronic signs of PEM. Histologically, chronic lesions of PEM and mild meningoencephalitis were observed.(2) In Argentina BoHV-5 infections also have the same characteristics as PEM. In a retrospective study from 1972 to 1999, of 89 cases previously diagnosed as PEM, 12 were caused by BHV-5 infections.(7)
In reports from other countries malacia of the cerebral cortex is absent or rarely reported.(2) Malacia of the cerebral cortex had also been reproduced in cattle inoculated with BoHV-5, but in most experimental reproductions of the disease this lesion is not reported. Nevertheless, some of these authors mentioned neuronal degeneration and necrosis or the presence of focal rarefaction necrosis. Deep malacia had not been reported in cases of BoHV-5 infections in other countries or in the experimental reproduction of the disease.(2)
The differential diagnosis of BoHV-5 infection in Brazil includes the different causes of PEM (sulphur intoxication, sodium chloride intoxication/water deprivation, lead intoxication, and thiamin deficiency). Other infectious diseases, like rabies, malignant catarrhal fever (MCF), and listeriosis, have to be included in the differential diagnosis list. In rabies, clinical signs and distribution of the lesionsmore frequently affect the spinal cord, brain stem and cerebellum, but some animals also show signs of cerebral involvement. Inclusion bodies are found in approximately 90% of the cases, mainly in the cerebellum, but also in brain stem, spinal cord and cerebrum. Some cases of MCF have signs of cerebral disease, but most also have keratitis and corneal opacity, as well as ulcerative lesions in the oral cavity and respiratory system. Listeriosis is a sporadic disease with characteristic lesions and signs affecting the brain stem.
JPC Diagnosis:
Conference Comment:
One reference text on veterinary neuropathology describes three features of inflammation within the central nervous system: 1) perivascular cuffing; 2) gliosis; and 3) neuronal satellitosis and neuronophagia.(9) Perivascular cuffing and extension of inflammatory cells into the adjacent neuropil is a significant histologic finding that should propel the pathologist to search for the underlying cause of the lesion. The leukocyte populations surrounding the vessels may offer clues as to the type of inflammatory process and associated etiology, e.g. lymphoplasmacytic for viral infections, suppurative for bacterial infections, etc.(9)
Gliosis is characterized by the increased prominence of glial cells due to hyperplasia, hypertrophy or both. Glial cells can: undergo transformation to macrophages; surround degenerating neurons (satellitosis); and begin phagocytosis of the neuron (neuronophagia).(5) Astrocytes also undergo reactive changes in response to tissue damage characterized by cell swelling, cytoplasmic eosinophilia, large vesiculate nuclei, and, rarely, multiple nuclei. Reactive astrocytes are frequently referred to as gemistocytes or gemistocytic astrocytes. In addition to the reactive astrocytic changes, there is often hyperplasia of astrocytes. As the severity of the lesion worsens, the number of astrocytes typically increases resulting in more pronounced and dense astrocytosis; these cells frequently remain at the affected site after the lesion is resolved (glial scar).(5)
The contributor provides a thorough discussion of the epidemiology, clinical presentation, pathogenesis, lesions and differential diagnosis list for BoHV-5 infection.
References:
2. David N, Hubner SO, Riet-Correa F, Halfen D, Lemos RA. Reactivation of latent bovine herpesvirus type 5 in cattle with polioencephalomalacia induced by ammonium sulphate. Pesq Vet Bras. 2007;27:445-441.
3. Elias F, Schild AL, Riet-Correa F. Meningoencefalite e encefalomalacia por herpes v+�-�rus bovino-5: distribui+�-�+�-�o das les+�μes no sistema nervoso central de bovinos naturalmente infectados. Pesq Vet Bras. 2004;24:123-131.
4. Engels M, Ackermann M. Pathogenesis of ruminant herpesvirus infections. Vet Microbiol. 1996;53:3-15.Â
5. Maxie MG, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 5th ed., vol. 1. Philadelphia, PA: Elsevier Ltd; 2007:292-295.
6. Perez SE, Bretschneider MR, Leunda MR, Osorio FA, Flores EF, Ode³n AC. Primary infection, latency, and reactivation of bovine herpesvirus type 5 in the bovine nervous system. Vet Pathol. 2002;39:437-444.
7. Perez SE, Vagnozzi A, Sur JH, Odriozola E, Campero CM, Ode³n AC. An+�-�lisis retrospectivo de casos con diagn³stico de necrosis cerebrocortical y su relaci³n com herpesvirus bovino tipo 5. Rev Arg Microbiol. 2003;35:69-73.
8. Silva AM, Weiblen R, Irigoyen LF, et al. Experimental infection of sheep with bovine herpesvirus type-5 (BHV-5): acute and latent infection. Vet Microbiol. 1999;66:89-99.Â
9. Summers BA, Cummings JF, de Lahunta A. Principles of neuropathology. In: Veterinary Neuropathology. St. Louis, MO: Mosby; 1995:39-42.