Signalment:
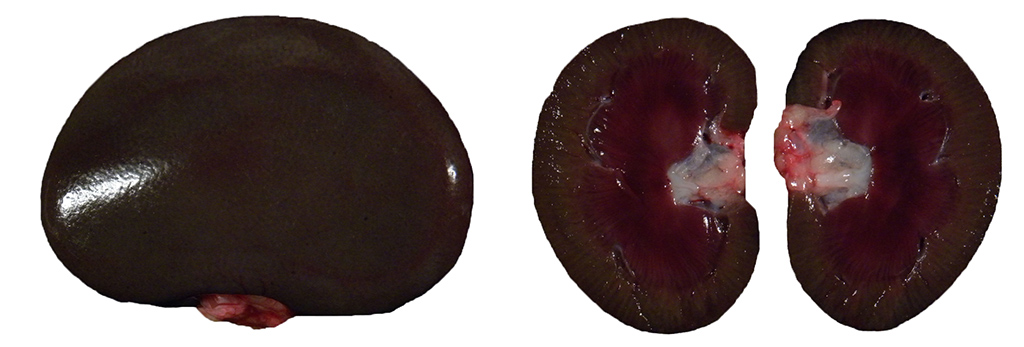
Gross Description:
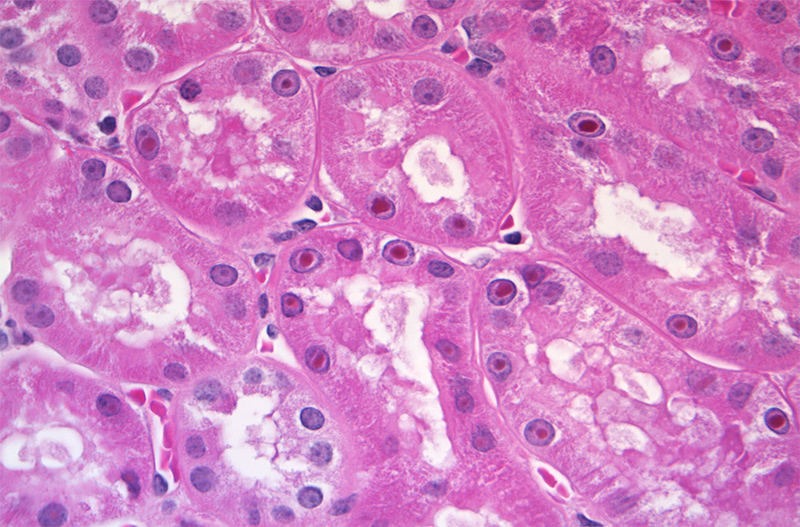
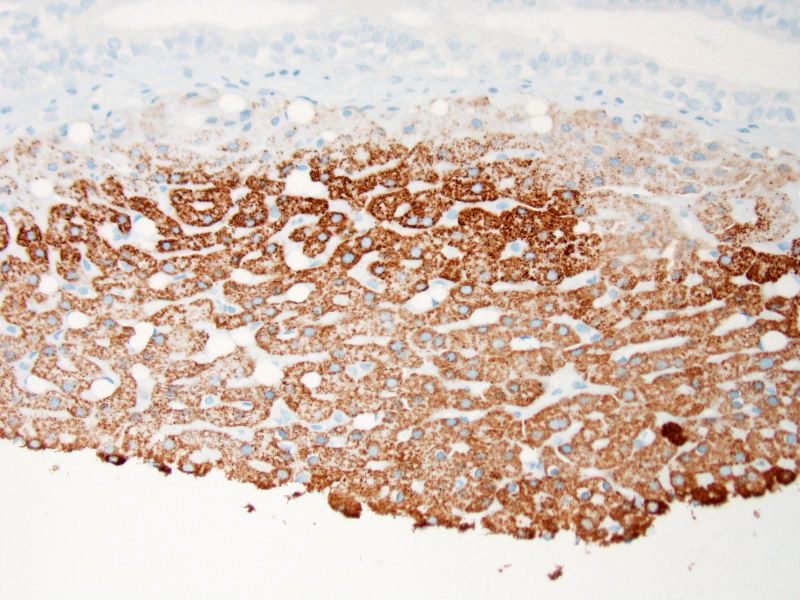
Histopathologic Description:
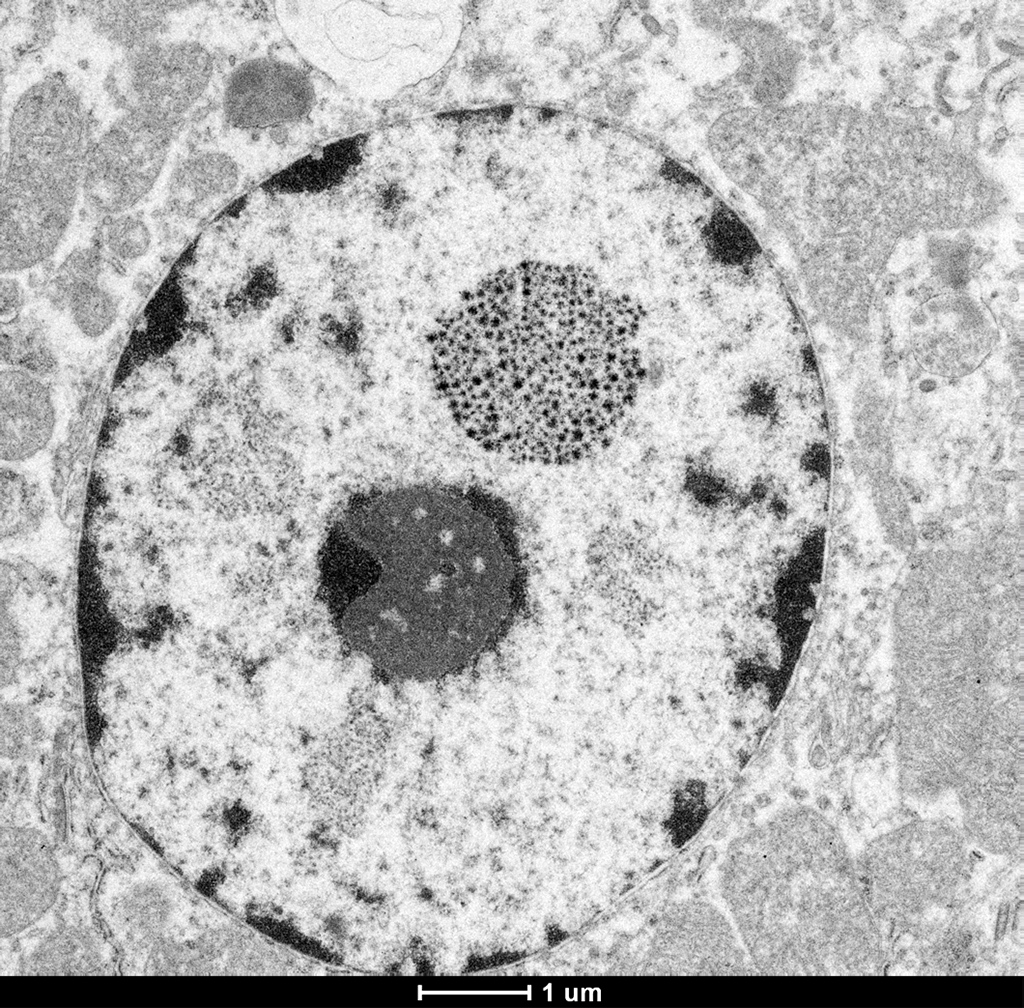
Electron microscopy (EM) revealed numerous renal cortical tubular epithelial cells that contained a single, variably sized intranuclear inclusion. The inclusions are composed of densely grouped, co-aggregated, electron-dense crystalline material which appeared variably star or asterisk-shaped. The inclusions did not peripheralize or displace the nucleolus and the remaining nuclear structures appeared normal.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Bismuth subsalicylate (BSS) is the primary ingredient in Pepto-Bismol (Proctor & Gamble, USA). It is estimated that roughly 99% of bismuth ingested is excreted in the feces.2 Bismuth levels in blood increase with chronic administration, but are not routinely associated with toxicity.2 Despite the low levels of bismuth absorbed and the minimal risk of toxicity, the literature discourages prolonged administration.2 The absorption of bismuth from the gastrointestinal tract may be increased under a variety of situations including patient variation, the formulation of the bismuth compound or salt, time of gastric emptying, alteration of gastric pH, and co-administration of bismuth with products or foodstuffs that contain thiol groups, cysteine, and/or fruit juice.1-7, 11 Bismuth is deposited in multiple organs, but is preferentially retained longer in the kidney when compared to other organs.10 Renal bismuth inclusions have been identified 1-30 years after parenteral treatment.10
The diagnosis of gross renal bismuth pigmentation and intranuclear bismuth inclusions in this macaque was based on the combination of a history of chronic BSS administration, elemental screening, gross and histologic findings, histochemical stain results, and electron microscopy.
Toxic element screening confirmed not only the presence of bismuth (110.6ppm), but that the levels were measured at 500-1000 times higher than normal acceptable bismuth levels. Based on literature published in wild game birds normal levels of bismuth in liver and muscle are <0.1ppm.5 Work performed in rats suggests that normal tissue levels for bismuth in kidneys are <0.2 ppm.1 The heavy metal screening also revealed levels of lead considered within normal limits disproving lead as the source of the intra-nuclear inclusions.
The dark green to black renal cortical pigmentation is consistent with pigmented bismuth compound accumulation. As the kidney is the documented primary target organ for bismuth accumulation, this was the most likely organ to exhibit pigmentation.10 There was no gross or histologic evidence of this pigment in any other organs. Other causes of grossly visible green black renal pigment such as hemozoin and melanin were disproved through microscopic appearance of intranuclear localization and patient history. The animal had lived in a non-endemic area for malaria-causing Plasmodium sp. throughout its lifetime and the animal had not received any blood or tissue transplants that could have resulted in iatrogenic transmission. Further, there was no histologic evidence of malaria, effectively ruling out hemozoin pigment.
A variety of histochemical stains were employed to help correlate the appearance of the intranuclear inclusions and intra-cytoplasmic granules observed in the renal tubular epithelium with the elemental screening results. Ziehl-Neelson acid fast stain is used primarily for the detection of acid-fast bacteria. However, lead inclusions are known to stain positive on paraffin embedded tissue, but less intensely when compared to fresh non-paraffin embedded samples.12 Compared to bismuth inclusions that stain acid-fast positive with frozen tissue and negative with paraffin embedded tissues.3 Perls Iron stain used to detect ferric iron in tissue was negative, determining that iron and iron-containing compounds such as ferritin were not present. Alizarin Red stain for calcium was also negative. To demonstrate that the material was intrinsically pigmented, unstained tissues were examined revealing brown inclusions. When unstained tissue sections were treated with a 10 second application of 30% hydrogen peroxide, the inclusions were no longer visible. The presumptive mechanism for decolorization with hydrogen peroxide is that the hydrogen peroxide oxidizes the bismuth sulfide resulting in formation of colorless bismuth sulfate.13 Melanin is resistant to decolorization with this short term hydrogen peroxide application.13 Fontana Masson and Churukian-Schenk, silver based stains used for the non-specific demonstration of melanin and argyrophilic granules, stained the inclusions brown-black. As the hydrogen peroxide treatment suggested that the material was not actually melanin, it was presumed that the bismuth salt inclusions possessed the ability to bind silver from a silver solution and reduce it to visible metallic silver with and without a reducing agent thus exhibiting both argentaffin and argyrophilic properties.
Electron microscopy confirmed the presence of an electron dense material consistent with heavy metal or mineral within the nucleus of proximal renal tubule epithelial cells. The appearance of bismuth inclusions are dependent upon the method of fixation, sections fixed with glutaraldehyde exhibit a homogenous electron dense appearance.4 However, inclusions in tissue fixed with osmium have a more granular and fibrillar appearance.4 The latter is more consistent with the inclusions we identified in our osmium fixed samples.
Increased absorption of bismuth in this macaque is thought to be related to a variety of factors including chronic vomiting that likely altered the time of gastric emptying. Additionally, concomitant therapeutics, famotidine and omeprazole, were frequently administered with the primary therapeutic BSS for this animals gastrointestinal disturbance. Famotidine is an H2 receptor antagonist. Work performed with other H2 receptor antagonists (ranitidine) has demonstrated an increased systemic absorption of tripotassium dicitrate bismuthate.8 Omeprazole, a proton pump inhibitor has also been linked to increased absorption of tripotassium dicitrate bismuthate.11 Although this animal was administered BSS, not tripotassium dicitrate bismuthate, this still suggests an additional potential for increased absorption. Furthermore, this animals diet of routine monkey chow contains normal dietary levels of cysteine, and as enrichment this animal routinely received whole fruits and fruit juices. Both cysteine because of its thiol group, and ascorbic acid derived from fruits and fruit juices have been linked to the formation of soluble bismuth in vivo and in vitro.7,10 The prolonged administration of BSS by itself or in conjunction with drugs that alter gastric pH, and routine administration of foodstuffs that contain thiol group compounds and ascorbic acid could have singly or in combination been responsible for increased systemic absorption of bismuth and consequently this animals gross and histologic findings.
JPC Diagnosis:
Conference Comment:
Conference participants briefly discussed other causes of intranuclear inclusion bodies, which included viral etiologies such as herpesviruses, adenovirus, and polyomavirus; other heavy metals, such as lead; and cytoplasmic invaginations. Likely due to the recent publication of this case in Veterinary Pathology, virtually every conference participant included BSS near the top of their list of differentials diagnoses for this lesion.6
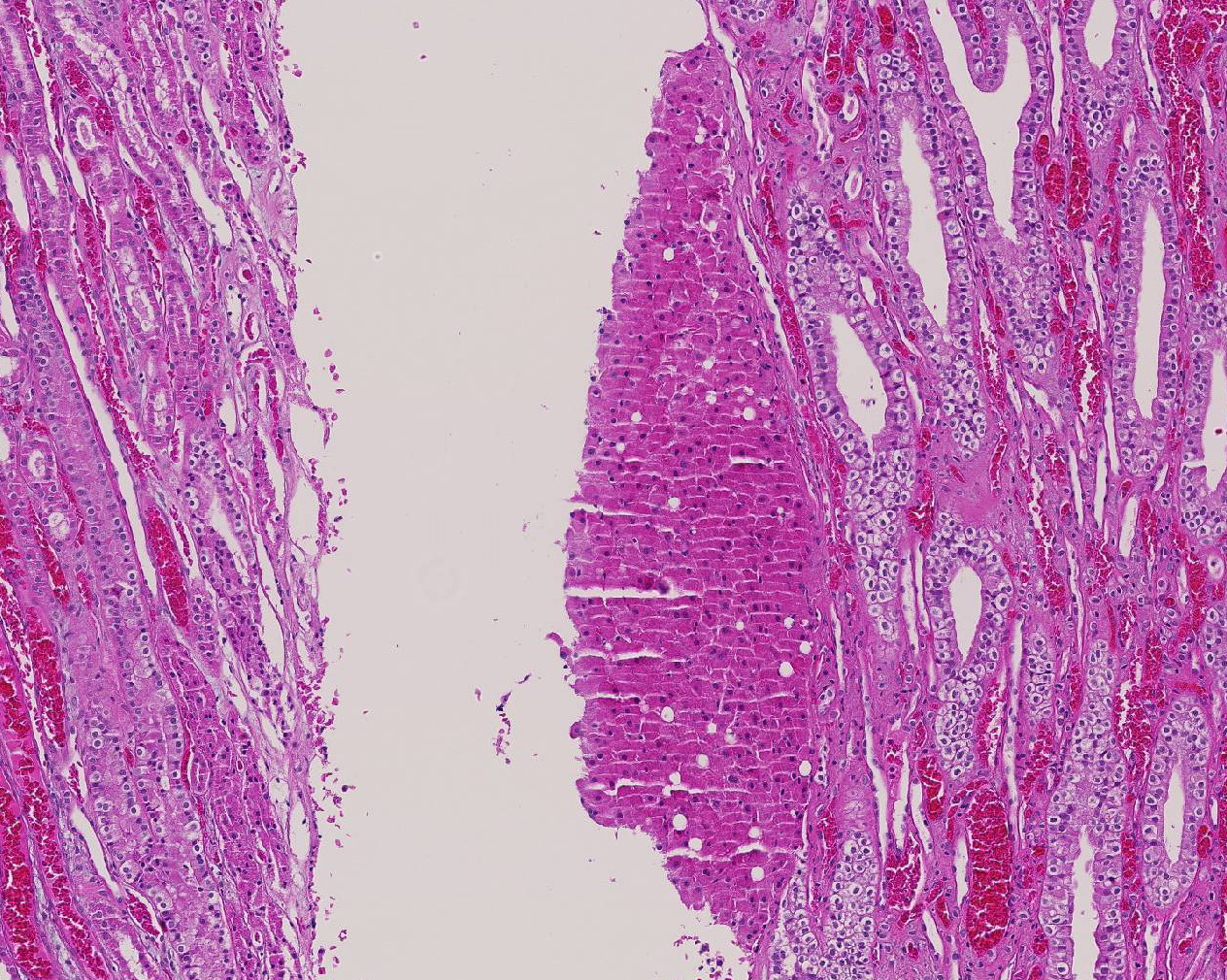
In addition to the intranuclear inclusions predominantly within the renal proximal convoluted tubules, the more perceptive conference participants also identified what appeared to be a 3 x 2 mm section of liver attached to the kidney near the renal pelvis. This small section of tissue demonstrated intense and specific intracytoplasmic immunoreactivity for hepatocyte antigen run by the Joint Pathology Center after the conference. Conference participants could not reach a consensus on whether this represented ectopic liver tissue (hepatic choristoma) or is simply a piece of liver that was dragged in during tissue processing. The tissue appears to be attached to the underlying renal parenchyma, but it is also present on a cut border, further adding to the disagreement. Hepatic choristomas have been typically reported as incidental findings in the kidney and various abdominal and extra-abdominal sites, with the gallbladder the most common location in humans. Other rare sites include the retroperitoneum and the adrenal gland. Ectopic liver has also been very rarely associated with the development of hepatocellular carcinoma.8 If this case represents a true hepatic choristoma in the kidney, it is of academic interest, but likely of no clinical significance to this animal.
References:
1. Allain P, Krari N, Chaleil D, et al. Effects of some chelating agents on bismuth absorption in the rat. Fundam Clin Pharmacol. 1991;5(1):39-45.
2. Bierer DW. Bismuth subsalicylate: history, chemistry, and safety. Rev Infect Dis. 1990;12(Suppl 1):3-8.
3. Burr RE, Gotto AM, Beaver DL. Isolation and analysis of renal bismuth inclusions. Toxicol Appl Pharmacol. 1965;7(4):588-91.
4. Ghadially FN, Young NK. Ultrastructural and x-ray analytical studies on intranuclear bismuth inclusions. Virchows Arch B Cell Pathol. 1976;(1):45-55.
5. Jayasinghe R, Tsuji LJ, Gough WA, et al. Determining the background levels of bismuth in tissues of wild game birds: A first step in addressing the environmental consequences of using bismuth shotshells. Environ Pollut. 2004;132(1):13-20.
6. Johnson AL, Blaine ET, Lewis AD. Renal pigmentation due to chronic bismuth administration in a rhesus macaque (Macaca mulatta). Vet Path. 2015; 52(3):576-579.
7. Mahony DE, Woods A, Eelman MD, et al. Interaction of bismuth subsalicylate with fruit juices, ascorbic acid, and thiol-containing substrates to produce soluble bismuth products active against Clostridium difficile. Antimicrob Agents Chemother. 2005;49(1):431-3.
8. Merve A, Scheimberg I. Ectopic liver tissue in the kidney: Case report and literature review. Pediatr Dev Pathol. 2014; 17(5):382-385.
9. Nwokolo CU, Prewett EJ, Sawyerr AM, et al. The effect of histamine H2-receptor blockade on bismuth absorption from three ulcer-healing compounds. Gastroenterology. 1991;101(4):889-94.
10. Slikkerveer A, de Wolff FA. Pharmacokinetics and toxicity of bismuth compounds. Med Toxicol Adverse Drug Exp. 1989; 4(5):303-23.
11. Treiber G, Walker S, Klotz U. Omeprazole-induced increase in the absorption of bismuth from tripotassium dicitrato bismuthate. Clin Pharmacol Ther. 1994;55(5):486-91.
12. Wachstein M. Studies on inclusion bodies; acid-fastness of nuclear inclusion bodies that are induced by ingestion of lead and bismuth. Am J Clin Pathol. 1949;19(7):608-14.
13. Wachstein M, Zak FG. Bismuth Pigmentation: Its Histochemical Identification. Am J Pathol. 1946 May;22(3):603-11.