Signalment:
Gross Description:
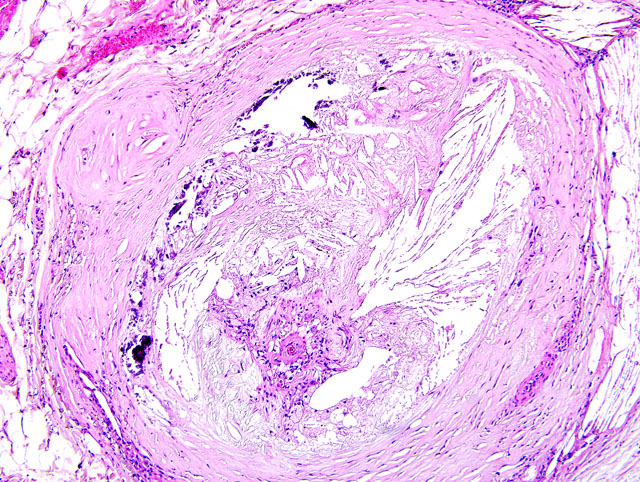
1. Systemic arteriosclerosis showing marked calcification in the arterial wall and luminal narrowing.
2. Thrombosis of the internal iliac, femoral, popliteal, and right superficial branchial arteries.
3. Atrophy of the pituitary and thyroid glands.
4. Dry gangrene in the tail, bilateral hind limbs and right forelimb.
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
JPC Diagnosis:
Conference Comment:
| Time | Light Microscope Findings |
| 0 to 0.5 hr | None |
| 0.5 to 4 hrs | None, or myofiber waviness at the border of the lesion |
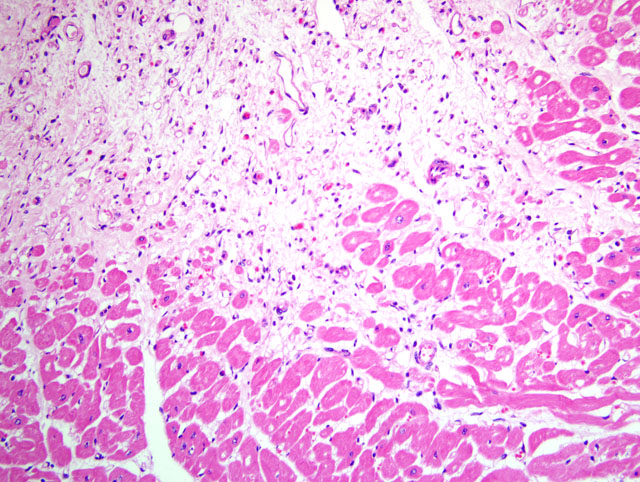
| 4 to 12 hrs | Early coagulation necrosis with hemorrhage and edema |
| 12 to 24 hrs | Coagulation necrosis; myocyte hypereosinophilia and pyknosis, contraction band necrosis; early neutrophilic infiltrate |
| 1 to 3 days | Coagulation necrosis; loss of cross striations and loss of nuclei; increased neutrophilic infiltrate |
| 3 to 7 days | Early myofibers disintrigration; necrosis of neutrophils; macrophages at the border of the lesion |
| 7 to 10 days | Increased macrophage infiltrate with phagocytosis of necrotic cells; early granulation tissue at infarct margins |
| 10 to 14 days | Well-developed granulation tissue |
| 2 to 8 weeks | Increased collagen deposition; decreased cellularity; regression of granulation tissue capillaries |
| >2 months | Dense collagenous scar |
In addition to hypothyroidism-induced hypercholesterolemia, diabetes mellitus is often associated with atherosclerosis in dogs. In one study, dogs with atherosclerosis were over 53 times more likely than dogs without atherosclerosis to have diabetes mellitus, and over 51 times more likely to have hypothyroidism than dogs without atherosclerosis, but were not more likely to have hyperadrenocorticism than dogs without athoersclerosis.(1) Dogs are otherwise considered relatively atheroresistant, as are cats, cattle, goats, and rats. While this condition is only rarely clinically significant in domestic animals, nonhuman primates and pigs are the most popular animal models of human disease. As atherosclerosis is a significant cause of human mortality, it merits considerable research interest. Pigs, rabbits, and chickens are atherosensitive, and develop the disease in response to high dietary cholesterol intake, particularly when the proportion of very low density lipoproteins (VLDL) is increased.(3)
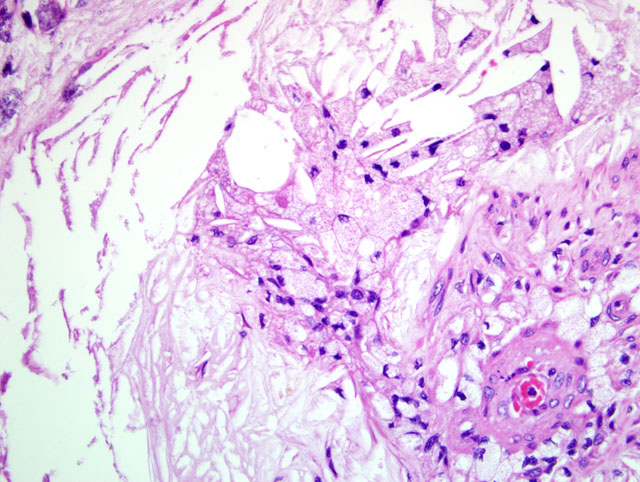
The pathogenesis of atherosclerosis in humans is well-studied, and the currently-accepted model, the response-to-injury hypothesis, characterizes atherosclerosis as a chronic response of the arterial wall to endothelial injury. An exhaustive review of the pathogenesis is beyond the scope of this report. Briefly, hypothyroidism or diabetes mellitus results in dyslipoproteinemia (i.e. increased low density lipoprotein (LDL) cholesterol, decreased high density lipoprotein (HDL) cholesterol, and/or increased levels of abnormal lipoprotein (a)). This causes endothelial damage by a variety of mechanisms, including oxygen free radical production that speeds the decay of nitric oxide and decreases its vasodilator activity. The damaged endothelium allows lipoproteins to accumulate in the intima, where they are oxidized by free radicals generated by macrophages and endothelial cells. Oxidized LDLs, which are directly toxic to endothelium and smooth muscle cells, are ingested by macrophages and smooth muscle cells, which become characteristic so-called foam cells. Damaged endothelium also allows the adhesion of platelets and expresses vascular cell adhesion molecule 1 (VCAM-1), which binds monocytes and T lymphocytes. Platelets and migrating leukocytes elaborate platelet-derived growth factor, interleukin-1, and interferon-gamma, which promote smooth muscle proliferation and extracellular matrix synthesis.(4) The resulting characteristic histologic lesion is the deposition of lipid in the vessel wall (i.e. atheroma), which is overlain by fibrosis and mineralization. The lesion in dogs differs from that of humans with respect to the location of lipid, which is primarily within the tunica media and adventitia in dogs, but primarily within the tunica intima in humans. With chronicity, atheromas may cause luminal narrowing and/or ulceration, leading to thrombosis, hemorrhage, or aneurysm.(3)
Most affected are the aorta, iliac, carotid, coronary, and femoral arteries. Grossly, affected vessels are enlarged and firm, with prominent yellow-brown nodules that project into the lumen. Other degenerative arterial diseases in domestic species include arteriosclerosis, and medial calcification, summarized in the table below.(6)
| Name | Clinical and Gross Findings | Histomorphology |
| Atherosclerosis | Pigs, rabbits, chickens are atherosensitive; dogs, cats, cattle, goats, and rats are atheroresistant; affects large elastic and large and medium muscular arteries; seen most commonly in dogs with hypothyroidism or diabetes mellitus; gross = vessel wall thickened and firm with yellow-brown nodules projecting into the lumen | Fibrofatty plaque or atheroma in the vessel wall (tunica intima and media) and lipid-laden macrophages and/or smooth muscle cells (i.e. "foam cells") in the media and intima |
| Arteriosclerosis | Occurs in many species but rarely clinically significant; age-related, degenerative change; means "arterial hardening"; results in loss of elasticity; abdominal aorta most frequently affected; also occurs at arterial branching sites (turbulent blood flow); gross = raised, firm, white plaques | Intimal thickening by mucopolysaccharides (early) or medial thickening by proliferating smooth muscle cells and fibrosis that infiltrates into the intima (later); splitting of the internal elastic lamina |
| Arterial medial calcification | Common; affects elastic and muscular arteries; caused by certain plant toxins (e.g. Cestrum diurnum, Solanum malacoxylon, Trisetum flavescens), vitamin D toxicosis, renal disease (aged guinea pigs and rats), and paratuberculosis; spontaneous in rabbits; gross = solid, pipe-like vessels with white, solid, raised intimal plaques | Prominent, granular, basophilic material on medial elastic fibers; form a circumferential ring of mineralization in muscular arteries |
In horses, intimal bodies in small arteries and arterioles, and siderocalcinosis of cerebral vessels are usually incidental findings of no clinical significance.(3,6)
References:
2. Kemppainen RJ, Clark TP: Etiopathogenesis of canine hypothyroidism. Vet Clin North Am Small Anim Pract 24:467-476, 1994
3. Maxie MG, Robinson WF: Cardiovascular system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 2, pp. 56-62. Elsevier Saunders, Philadelphia, PA, 2007
4. Mitchell RN, Schoen FJ: Blood vessels. In: Robbins and Cotran Pathologic Basis of Disease, eds. Kumar V, Abbas AK, Fausto N, Aster JC, 8th ed., pp. 496-504. Saunders Elsevier, Philadelphia, PA, 2010
5. Schoen FJ, Mitchell RN: The heart. In: Robbins and Cotran Pathologic Basis of Disease, eds. Kumar V, Abbas AK, Fausto N, Aster JC, 8th ed., pp. 547-559. Saunders Elsevier, Philadelphia, PA, 2010
6. Van Vleet JF, Ferrans VJ: Cardiovascular system. In: Pathologic Basis of Veterinary Disease, eds. McGavin MD, Zachary JF, 4th ed., pp. 599-602. Mosby Elsevier, St. Louis, MO, 2007