Signalment:
Adult, female, transgenic β-actin luciferase mouse on an FVB strain background (
Mus musculus).Animals were supplied by a commercial vendor and held as stock animals with routine husbandry for approximately 1 month without undergoing any procedure or treatment. Animals were found during mornin g health checks to be non-responsive with a hunched posture, and were subsequently euthanized. Four stock animals in this batch were found moribund within a 48 hour period. Clinical signs recorded in animals included a hunched posture, piloerection, dehydration and inappetance, a reddish nasal discharge, and a wet chin and chest.
Gross Description:
Nosignificantabnormalitieswerenoted at necropsy in animals submitted from this batch.
Histopathologic Description:
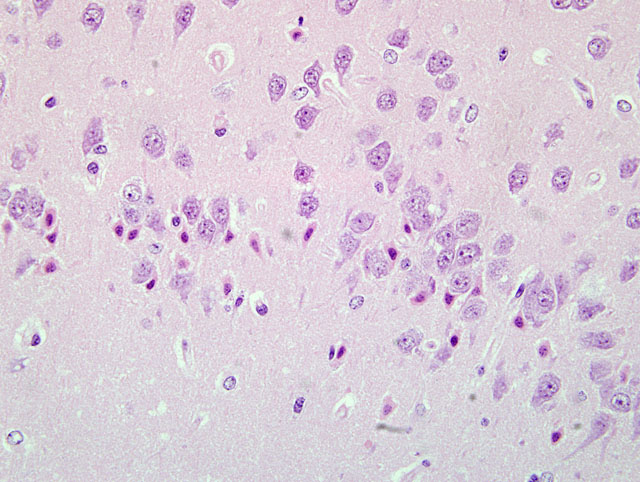
Sections of the brain show multifocal acute neuronal necrosis in many areas. Necrotic neurons are shrunken and angular with pyknotic nuclei and red cytoplasm. Necrotic neurons can be found scattered in most nuclei; however, regions of the cerebral cortex, thalamus and hippocampus are severely affected, but the distribution varies between individuals. Half of the affected animals from this batch additionally had multifocal random areas of hepatic coagulative necrosis.
Morphologic Diagnosis:
Brain: Severe acute neuronal necrosis.
Condition:
"Space cadet" mouse
Contributor Comment:
The presentation of these animals clinically, associated with lesions of widespread neuronal necrosis and occasional hepatic coagulative necrosis, is classic for a strain related entity in FVB mice reported as lethal epileptic syndrome or -�-�Space cadet syndrome.(1,2) The FVB is an inbred mouse strain used extensively in transgenics research because of its superior reproductive performance and large pronuclei which facilitate microinjection of genomic material.(2) However, the strain is recognized as seizure prone, with both spontaneous and induced seizures (tail tattooing, fur clipping, and audiogenic) being reported.(2) Additionally, FVB mice are more prone to neuronal death with kainate-induced seizures, compared with C57Bl/6 mice, even with seizures of equal duration and intensity scores.(8) In some colonies, spontaneous mortality rates in animals 4-12 months of age may approach 20%, with many investigators reporting that female mice are more prone to seizures than males.(1,2,6) Observed seizures include facial grimace, chewing automatism, ptyalism with matting of ventral neck and forelimbs, and clonic convulsions that may progress to tonic convulsions and death.(2) When seizures are not observed clinical signs are generally non-specific, including lethargy, moribundity, matting of fur, or being found dead.(2) Syndrome-associated histologic lesions include neuronal necrosis in the cerebral cortex, hippocampus and thalamus; astrocytosis, gliosis, and poliomalacia are reported in animals with a longer post-seizure survival interval.(1,2) Acute coagulative necrosis of hepatocytes has also been reported, which Goelz et al. interpret as resulting from terminal hypoxia in seizuring animals.(2)
The neuronal necrosis observed in these mice is reminiscent of that observed in cases of status epilepticus. Excitotoxicity is a proposed mechanism of neuronal death in status epilepticus, though hyperthermia, hypoxia, hypotension and hypoglycaemia associated with prolonged seizuring may exacerbate brain injury.(5) Excitotoxic neuronal death is caused by elevated levels of excitatory neurotransmitters, particularly glutamate, with the subsequent opening of the glutamate receptor-associated ion channels causing prolonged depolarization of neurons and increased intracellular calcium levels which lead to cell death.(7) The cause of FVB epileptic syndrome has not been determined to our knowledge, although genes involved in hippocampal excitability, hyperexcitability and glutamate release have been proposed as likely candidates.(4) Work by Schauwecker et al has determined that the susceptibility of FVB mice compared to C57Bl/6 mice to seizure-associated neuronal death is linked to the galanin receptor 1 (GalR1) gene.(8) FVB mice have increased levels of GalR1 in the hippocampus by quantitative real time PCR relative to C57Bl/6 mice, although these authors report no polymorphisms between the two mouse strains in the regions of the gene analyzed.4 GalR1 and its ligand, the neuropeptide galanin, reduce seizure threshold by modulating the excitatory tone in the hippocampus by a hyperpolarizing action, and can inhibit the excitatory neurotransmitter glutamate.(7) GalR1 null mutation mice have spontaneous seizures and enhanced susceptibility to excitotoxin-induced neuronal injury.(4,7) The relationship of increased GalR1 to the mechanisms of seizure susceptibility and increased neuronal death FVB mice is undetermined, though it may reflect a compensatory change to increased excitatory neurotransmitter levels or increased excitatory tone in the hippocampus.
JPC Diagnosis:
Brain,cerebralcortex,hippocampus,and pyriform plexus: Neuronal necrosis, acute, multifocally extensive.
Conference Comment:
The moderator and conference participants commented on the well-preserved sections of brain with minimal tissue artifact. The moderator observed that this case presents an excellent example of neuronal necrosis. A focal point of discussion was differentiation between dark neurons and necrotic neurons. Dark neurons have been reproducibly induced by exerting pressure on fresh, unfixed tissues (i.e. post-mortem manipulation) or inadequate perfusion-fixation. The histologic characteristics of dark neurons are contracted, darkly stained neurons with an indistinct nucleus and a corkscrew-shaped apical dendrite.(3) In contrast, necrotic neurons, colloquially characterized as red and dead, are shrunken and angular with pyknosis or karyorrhexis and vacuolization of the surrounding neuropil.
Differentiation of dark neurons from necrotic neurons can be extremely difficult, especially considering some conditions causing acute neuronal necrosis, e.g hypoglycemia, status epilepticus and ischemia reperfusion can appear histologically similar.(3) Knowledge of post-mortem tissue handling, circumstances surrounding the animals death, and expected lesions based on historical data of previous toxicologic studies provide a clearer interpretation of the lesion and corroborative evidence. The chart below outlines some of the histologic differences and corroborative evidence to help distinguish between the two:
| Dark Neurons | Necrotic Neurons |
- Appear contracted, darkly stained neurons with an indistinct nucleus and a corkscrew- shaped apical dendrite
- Collapsed brain microvessels indicating poor or inadequate perfusion-fixation
- Consistent histomorpholgic features among all affected neurons
- No reported clinical signs
|
- Appear shrunken and angular with pyknosis or karyorrhexis and vacuolization of the surrounding neuropil
- Sequence of degradative changes indicating the changes occurred at different times
- Evidence of an inflammatory response: microglial cells, reactive astrocytes, etc.
- Associated clinical signs
|
References:
1. Donnelly TM. Whats your diagnosis: -�-�Space cadet syndrome in female FVB/n mice.Â
Lab An. 2007;36:16.Â
2. Goelz MF, Mahler J, Harry J, et al. Neuropathologic findings associated with seizures in FVB Mice.Â
Lab An Sc. 1998;48:34-37.
3. Jortner BS. The return of the dark neuron. A histological artifact complicating contemporary neurotoxicologic evaluation.
Neurotoxicology. 2006;27:628-634.Â
4. Kong S, Lorenzana A, Deng Q, McNeill TH, Schauwecker PE. Variation in Galr1 expression determines susceptibility to excitotoxin-induced cell death in mice.Â
Genes, Brain, Behav. 2008;7:587-598.
5. Lowenstein DH, Alldredge BK. Status epilepticus.Â
N Engl J Med. 1998;338:970-976.Â
6. Mahler JF, Stokes W, Mann PC, Takaoka M, Maronpot RR. Spontaneous lesions in aging FVB/N mice.Â
Toxicol Pathol. 1996;24:710-716.
7. Mitsukawa K, Lu X, Bartfai T. Galanin, galanin receptors and drug targets.Â
Cell Mol Life Sci. 2008;65:1796-1805.
8. Schauwecker PE. Genetic basis of kainate-induced excitotoxicity in mice: phenotypic modulation of seizure- induced cell death.Â
Epilepsy Res. 2003;55:201-210.