WSC 22-23:
Conference 4 Case 3
Signalment:
Adult (unknown age), female, Brazilian hedgehog/Brazilian porcupine (Coendou prehensilis)
History:
This porcupine was captured in a transitional region (native forest area on the outskirts of a small town) and sent to the Veterinary Hospital (UFMT) quite dehydrated and with swollen and wrinkled skin on its head. The animal received support treatment, but died the next day.
Gross Pathology:
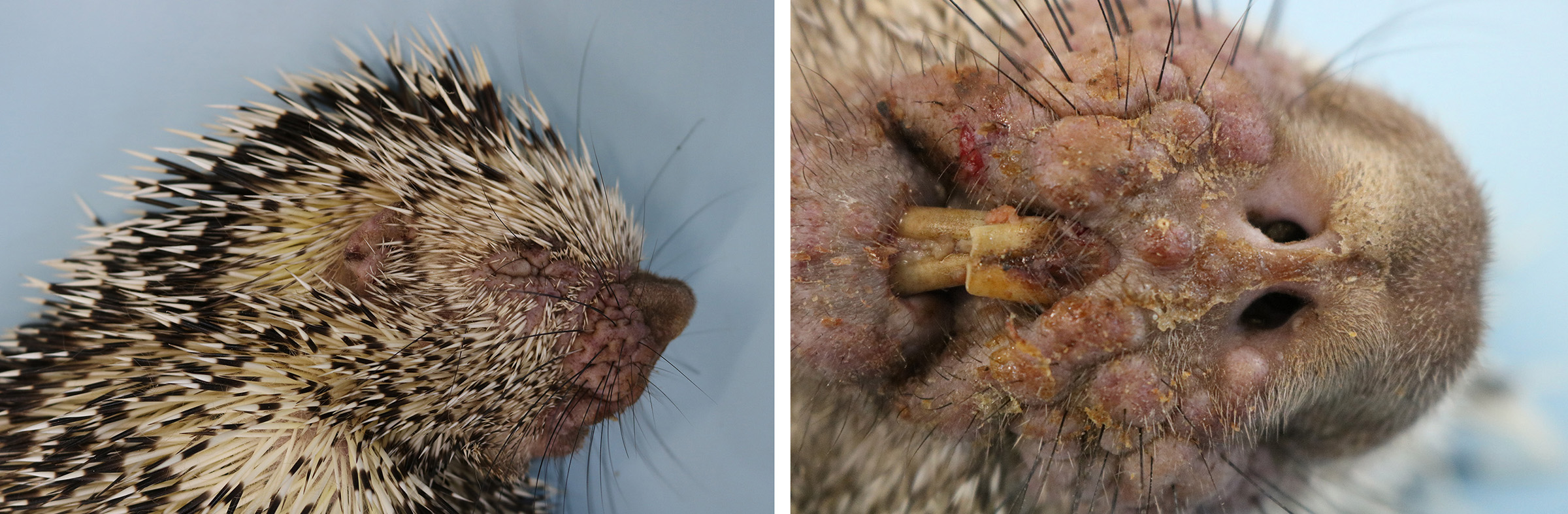
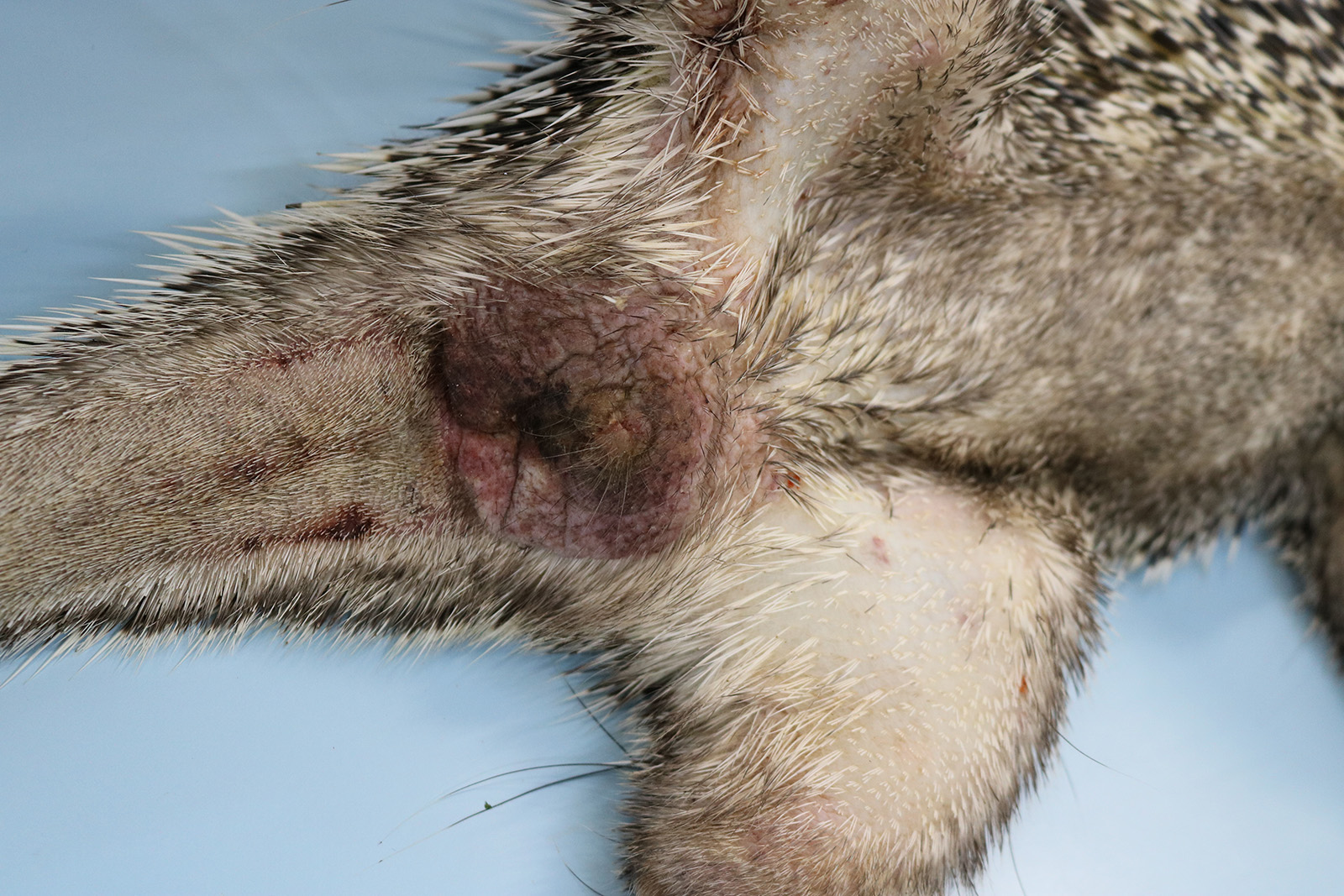
The animal was submitted to necropsy and showed good conservation status. On the skin of the head (ear, periocular region, lateral face, muzzle and submandibular region), and in the perigenital region there were papules with irregular coalescing plaques, separated by superficial to deep grooves and moderately erythematous. These changes in the periocular region caused eyelid slit occlusion, blocking the view of the eyeball. Additionally, in the nasal and perioral plane region, there were yellowish crusts and a slight superficial erosion of the skin. In the internal examination, no significant alterations were observed.
Laboratory Results:
A pan-pox universal PCR practice was performed 9 and subsequent amplicom sequencing, revealing 100% identity with the Brazilian porcupinepox virus (BPoPV).
Microscopic Description:
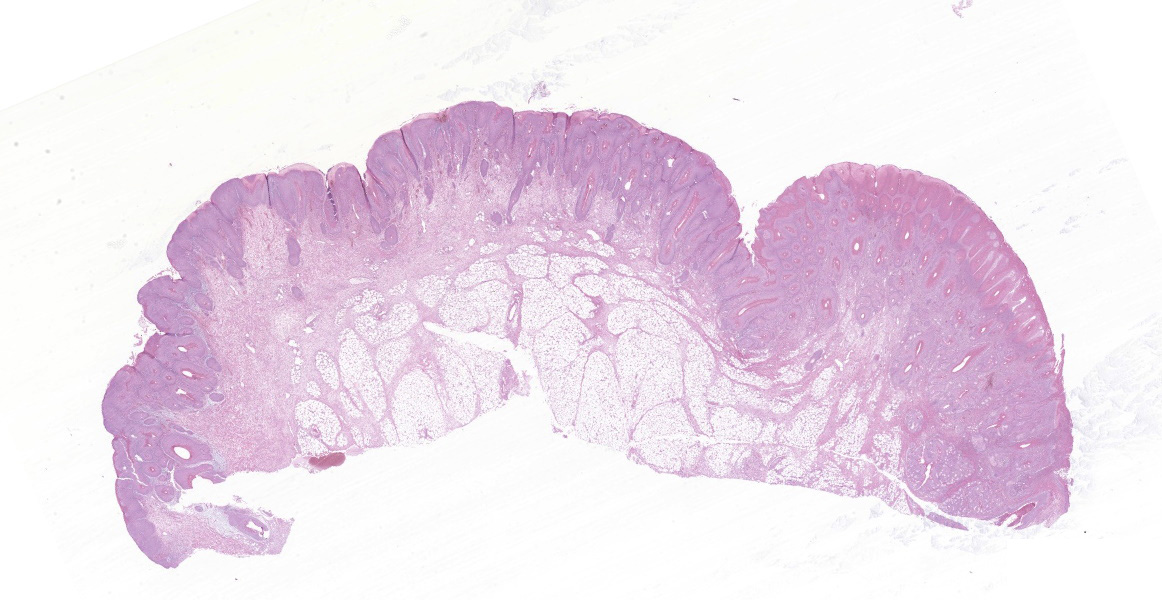
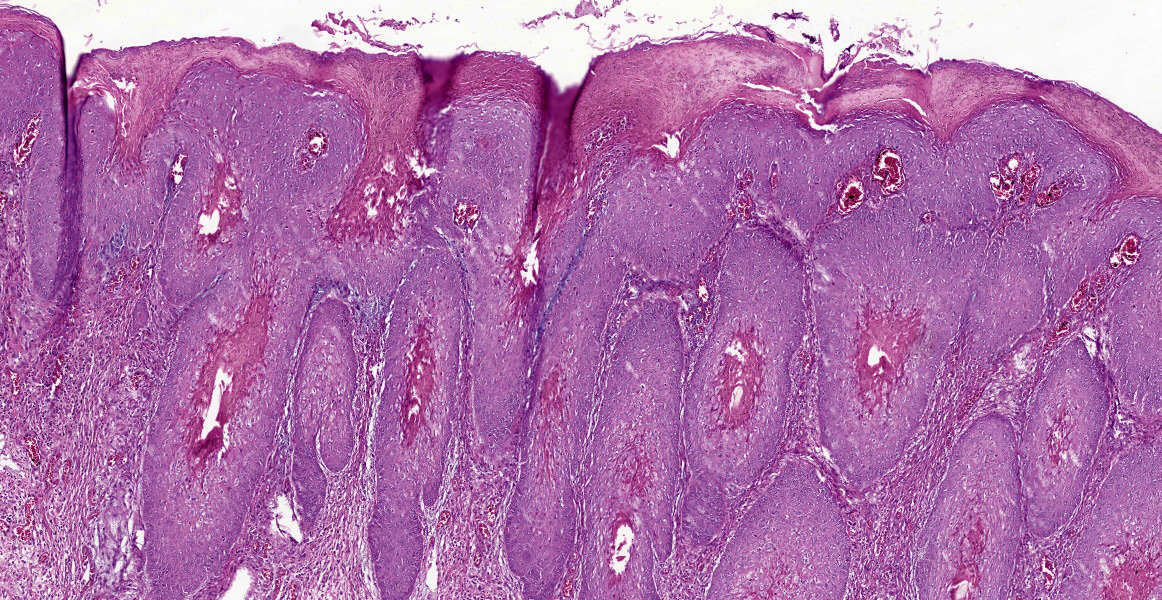
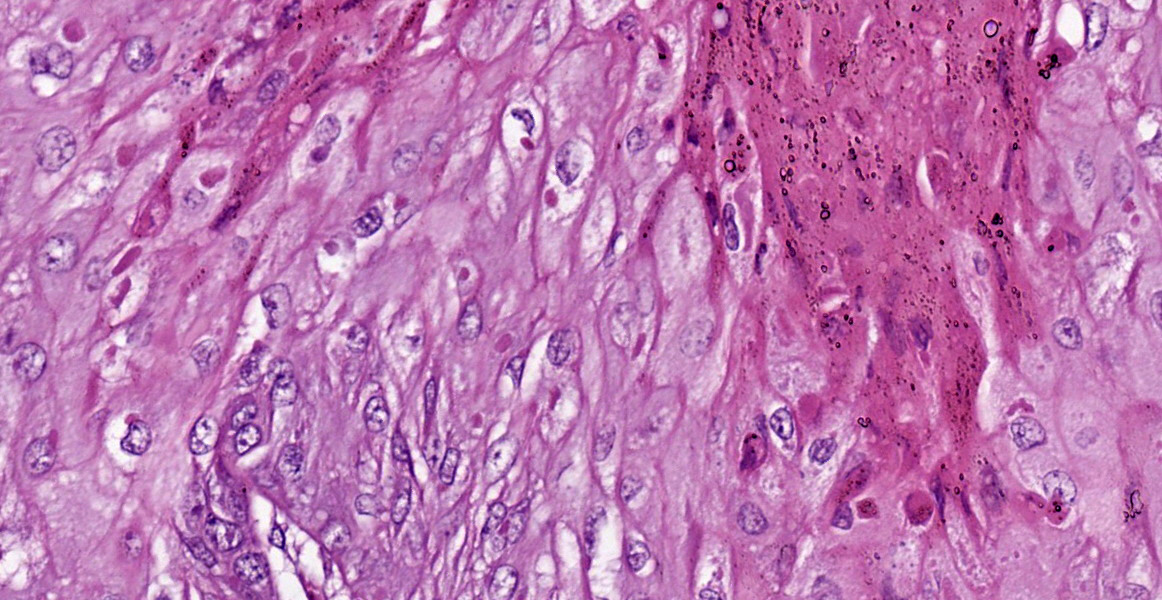
In the skin section, there are epidermic and hair follicles epithelial sheath with intense proliferation and thickening (acanthosis). In the epidermis, interdigitations towards the dermis (rete pegs) are frequent. Multifocally keratinocytes show intracytoplasmic edema and vacuolization (ballooning degeneration). Intercellular edema and mild to moderate, random, multifocal acantholysis are also seen, mainly at the basal and spinous layers, and are often keratinocytes with round/oval, eosinophilic, intracytoplasmic inclusion (Bollinger bodies). In the epidermis and hair follicles, there are multifocal areas of necrosis associated with moderate to severe neutrophil infiltration. There is a diffuse and moderate thickening of the stratum corneum in the epidermis, in which cell contours and finely elongated nuclei are seen, interspersed or paved with basophilic coccoid bacterial colonies (Brown & Hopps methods: gram positive), associated with cellular debris of neutrophils and keratinocytes. In the dermis, from the dermic-epidermic junction to the adipose panniculus there is an infiltration composed of lymphocytes, plasma cells and in multifocal to coalescent areas along with mononucleated inflammatory cells that present distended and eosinophilic cytoplasm with peripheral nucleus. Connective tissue fibers are separated (edema) with a discrete myxoid appearance, especially in the superficial dermis.
Contributor’s Morphologic Diagnoses:
- Hairy skin, vulva: Proliferative, necrotic, multifocal to coalescent epidermitis with parakeratotic hyperkeratosis, intracytoplasmic inclusion bodies in keratinocytes and intraepidermal and follicular pustules.
- Hairy skin, vulva: Diffuse, accentuated, and subacute lymphohistiocytic dermatitis.
Contributor’s Comment:
Poxvirus is one of the largest and most complex known viruses. It is a double-stranded DNA virus which comprises a wide variety of susceptible species (vertebrates and invertebrates). Historically, it has as its main representative the Variola virus (VARV), which causes human smallpox, the most devastating infectious disease in history.3,11 In the present case, the morphological findings observed in this Brazilian porcupine (Coendou prehensilis) are consistent with an infection caused by a new genus of poxviruses belonging to the family Poxviridae, subfamily Chordopoxvirinae. The first report of this infection occurred in 2019 in Brazil, in a free-living Brazilian porcupine that had lesions characteristic of poxvirus infection (There are approximately 1000 km of distance between the case reported by Hora in 2019 and this case). As it is phylogenetically distinct from the other poxviruses previously reported, this poxvirus was named Brazilian Porcupinepox Virus (BPoPV).6
Brazilian porcupine (OC) is an arboreal rodent belonging to the Erethizontidae family, found in forests and riparian areas in several South American countries. This animal is more frequently observed in Brazil, where in the last two years have been reports of OC showing characteristic clinical signs of poxvirus infection, associated with high mortality. This situation increased the alert regarding the risk and threat to the conservation of this species.6
Macroscopically, the lesions are characterized by wrinkling, thickening and skin erythema of the head, limbs, and genitals.6 Initially, these lesions appear as papules that coalesce and transform into plaques. Microscopically, the lesions occur mainly at the mucocutaneous junction, and are characterized by proliferative (hyperplasia and hyperkeratosis) and degenerative (ballooning degeneration and intercellular edema) changes in keratinocytes with visualization of intracytoplasmic eosinophilic viral inclusions.6 These macroscopic and microscopic changes were also seen in this case and are typically related to poxvirus infection.11
Other rodents and also lagomorphs are also susceptible to poxvirus infections, but with clinical manifestations, different from those presented by porcupines. Among them, myxomatosis in rabbits stands out, a fatal disease caused by the myxoma virus (MYXV), a Leporipoxvirus belonging to the Poxviridae family that is antigenically associated with the rabbit fibroma virus. 1,4 It is characterized by the presence of cutaneous nodules (myxomas) around the eyes, nose, mouth, ears and genitalia. It can also present in the amyxomatous (respiratory) form, with different degrees of severity.1,6 Microscopically, skin lesions consist of proliferation of stellate mesenchymal cells surrounded by a mucinous matrix. Intracytoplasmic inclusions are also observed in various cell types, as well as hyperplasia and/or degeneration in keratinocytes.8
Another member of the Poxviridae family is the rabbit fibroma virus (Shope), initially considered a benign disease, however, it can cause high morbidity and mortality in newborn animals. It is characterized by flat, mobile subcutaneous nodules that occur mainly on the limbs, feet, ears, muzzle and around the eyes, regressing spontaneously within a few months. Microscopic examination of skin lesions reveals proliferation of mesenchymal cells that become stellate or ovoid with multifocal areas of necrosis and inflammatory infiltrates of mononuclear and polymorphonuclear cells.8
Squirrel fibromatosis (SF) is caused by a poxvirus of the genus Leporipoxvirus, subfamily Chordopoxvirinae, and is also closely associated with rabbit fibroma virus. It has been described in red squirrels (Tamiasciurus hudsonicus), gray squirrels (Sciurus carolinensis) and fox squirrels (Sciurus niger). Macroscopically, the disease is characterized by firm, alopecic dermal nodules. Microscopically, typical poxviral lesions, epidermal hyperplasia, ballooning degeneration and intracytoplasmic inclusion bodies are observed, and the proliferation of atypical fibroblasts in the superficial dermis makes it distinct from other infections.2
It has been reported in pygmy mice proliferative epidermal lesions on the tail and paws caused by a poxvirus. Lesions appeared as firm, irregular, and pedunculated masses. On microscopic examination, the epidermis presents orthokeratotic and parakeratotic hyperkeratosis, ballooning degeneration of keratinocytes, and intracytoplasmic eosinophilic viral inclusions. Molecular analyzes identified that this poxvirus did not belong to any recognized genus until March 2018, this new species being called Brazospox virus,5 therefore molecular methods play a fundamental role in the identification and monitoring of the propagation of new poxviruses in wild animals, allowing warnings about the risk and emergence of diseases that represent risks to the conservation of species, especially those that are threatened.
Contributing Institution:
Universidade Federal de Mato Grosso, Faculdade de Medicina Veterinária, Hospital Veterinário, Laboratório de Patologia Veterinária, https:// www.ufmt.br/ufmt/site/, Av. Fernando Corrêa da Costa, nº 2367 - Bairro Boa Esperança. Cuiabá, Brasil - MT - 78060-900
JPC Diagnosis:
Haired skin, epithelium: Hyperplasia, diffuse, severe, with ballooning degeneration, necrosis, and intracytoplasmic viral inclusions.
JPC Comment:
The contributor provides both an informative example of the novel Brazilian porcupine poxvirus and a broad overview of the Leporipoxvirus genus. These viruses all belong to the subfamily Chordopoxvirinae, or poxviruses which infect vertebrates.6 There are ten genera and several newer unclassified viruses within this subfamily.6 The Orthopoxvirus genus contains several poxviruses which are zoonotic or pathogenic to humans, including both the historically devastating and now eradicated variola virus (smallpox) and the newer emerging public health threat, monkeypox virus.6, 12, 16
Smallpox has affected the human population for millennia, with evidence of smallpox lesions in Egyptian mummies from 1186-1070 BC.12 Smallpox is estimated to have caused 400,000 deaths annually in Europe in the eighteenth century and up to 4 million deaths in the Aztec and Incan empires after introduction of the virus to the Americas.12 Initial vaccines were developed in the eighteenth century, and after a widespread eradication campaign, the World Health Organization declared the disease eradicated in the late 1970s.16 Importantly, smallpox has a very narrow host range and lacks an animal reservoir, which made it susceptible to eradication strategies and contrasts with the wide host range of monkeypox virus.12
Monkeypox virus was first identified in cynomologous macaques imported from Africa into Copenhagen, Denmark in 1958.12, 16 This discovery lead to the somewhat inaccurate naming of the virus, whose largest known reservoir is rodents.16 Monkeypox infections have been documented in a wide range of animals, including numerous species of mice and rats, prairie dogs, hedgehogs, opossums, wild boar, several monkey and macaque species, organutans, chimpanzees, and gorillas.12 The first report of infection in a human occurred in 1970 in the Democratic Republic of Congo (previously Zaire). Humans can be infected from direct or indirect contact with animals, such as bites, scratches, and the hunting of small animals for bushmeat.16
After 1970, case rates slowly increased in Central and West Africa.16 Some of this spread is attributed to waning population immunity after termination of smallpox vaccination campaigns, which had offered cross protection against other orthopoxviruses; other factors include increased interaction with wildlife, civil unrest, and population dynamics.16 Sporadic outbreaks outside of Africa occurred due to import of infected animals (United States in 2003) and international travel (UK and Israel in 2018).16
The current monkeypox outbreak began in May 2022 and has been designated as a public health emergency of international concern by the World Health Organization.1, 18 As of September 2, 2022, 51,163 laboratory confirmed cases and 17 deaths have been reported in 102 countries around the world.1 Transmission occurs through large respiratory droplets and direct contact with skin lesions, and over 95% of reported cases are in men who have sex with men.1, 18 Clinical signs include fever and lymphadenopathy, with painful skin lesions such as papules, pustules, and ulcers most commonly found in the anogenital area, trunk and limbs, or face.18
In the current outbreak, only one case of monkeypox to date has been reported in a companion animal.14,17 A four year old male Italian greyhound living in a household with two humans infected monkeypox developed mucocutaneous pustules and ulcers.17 Swabs from the skin lesions were PCR positive for monkeypox and showed 100% homology with the virus isolated from one of the human owners.17
References:
- 2022 Monkeypox Outbreak: Global Trends. World Health Organization. September 2, 2022. Accessed September 4, 2022. https://worldhealthorg.shinyapps.io/mpx_global/
- Abade dos Santos, FA; Carvalho, CL; Pinto, A. et al. Detection of Recombinant Hare Myxoma Virus in Wild Rabbits ( Oryctolagus cuniculus algirus). Viruses. 2020, 12:1127.
- Bangari DS, Miller MA, Stevenson GW et al. Cutaneous and systemic poxviral disease in red (Tamiasciurus hudsonicus) and gray (Sciurus carolinensis) squirrels. Vet Pathol. 2009,46(4):667-72.
- Barrett, JW, McFadden, G. Origin and Evolution of Poxviruses. In: Domingo, E, Parrish, CR, Holland, JJ. Origin and Evolution of Viruses. Academic Press. 2008:431-446.
- Bertagnoli S, Marchandeau S. Myxomatosis. Rev Sci Tech. 2015; 34(2):549-56.
- Delhon GA. Poxviridae. In: MacLachlan NJ, Dubovi AJ, eds. Fenner’s Veterinary Virology. 5th New York, NY: Elsevier. 2017; 157-174.
- Hodo CL, Mauldin MR, Light JE et al. Novel Poxvirus in Proliferative Lesions of Wild Rodents in East Central Texas, USA. Emerg Infect Dis. 2018;24(6):1069-1072.
- Hora AS, Taniwaki SA, Martins NB, et al. Genomic Analysis of Novel Poxvirus Brazilian Porcupinepox Virus, Brazil, 2019. Emerg Infect Dis. 2021:(4): 1177-1180.
- Hughes AL, Irausquin S, Friedman R. The evolutionary biology of poxviruses. Infect Genet Evol. 2010 Jan;10(1):50-9.
- Krogstad AP, Simpson JE, Korte SW. Viral diseases of the rabbit. Vet Clin North Am Exot Anim Pract. 2005;8(1):123-38.
- Li Y, Meyer H, Zhao H, et al. GC content-based pan-pox universal PCR assays for poxvirus detection. J Clin Microbiol. 2010;48(1):268-276.
- MacNeill AL. Comparative Pathology of Zoonotic Orthopoxviruses. Pathogens. 2022; 11(8):892-914.
- Marinho-Filho, J., & Emmons, L. 2016. Coendou prehensilis. The IUCN Red List of Threatened Species2016: e.T101228458A22214580. https://dx.doi.org/10.2305/IUCN.UK.2016-2.RLTS.T101228458A22214580.en. Downloaded on 28 July 2021.
- Monkeypox in Animals. Centers for Disease Control and Prevention. August 17, 2022. Accessed September 4, 2022. https://www.cdc.gov/poxvirus/monkeypox/veterinarian/monkeypox-in-animals.html
- Oliveira JS, Figueiredo PO, Costa GB, et al. Vaccinia Virus Natural Infections in: Brazil: The Good, the Bad, and the Ugly. Vírus. 2017; 9(11):340.
- Peterson E, Koopmans M, Yinka-Ogunleye A, Ihekweazu C, Zumla A. Human Monkeypox Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect Dis Clin N Am. 2019; 33: 1027-1043.
- Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. The Lancet. 2022; 500(10353):658-659.
- Thornhill JP, Barkati S, Walmsley S, et a. Monkeypox Virus Infection in Humans across 16 Countries – April-June 2022. The New England Journal of Medicine. 2022;387(8):679-691.