Wednesday Slide Conference 2023-2024, Conference 3 Case 3
Signalment:
11-year-old, spayed female bassett hound, dog (canis familiaris)
History:
The patient was observed over a period of several months to develop a fluctuant soft tissue swelling associated with the upper eyelid, which was indicated to be subconjunctival/ subcutaneous with extension posteriorly towards the orbit. Interpretation of a fine needle aspirate was consistent with a neoplasm of epithelial origin. The entire submitted tissue was expressible through a small conjunctival incision over the mass at surgery, and the submitting veterinarian described a tissue with the gross appearance of fat.
Gross Pathology:
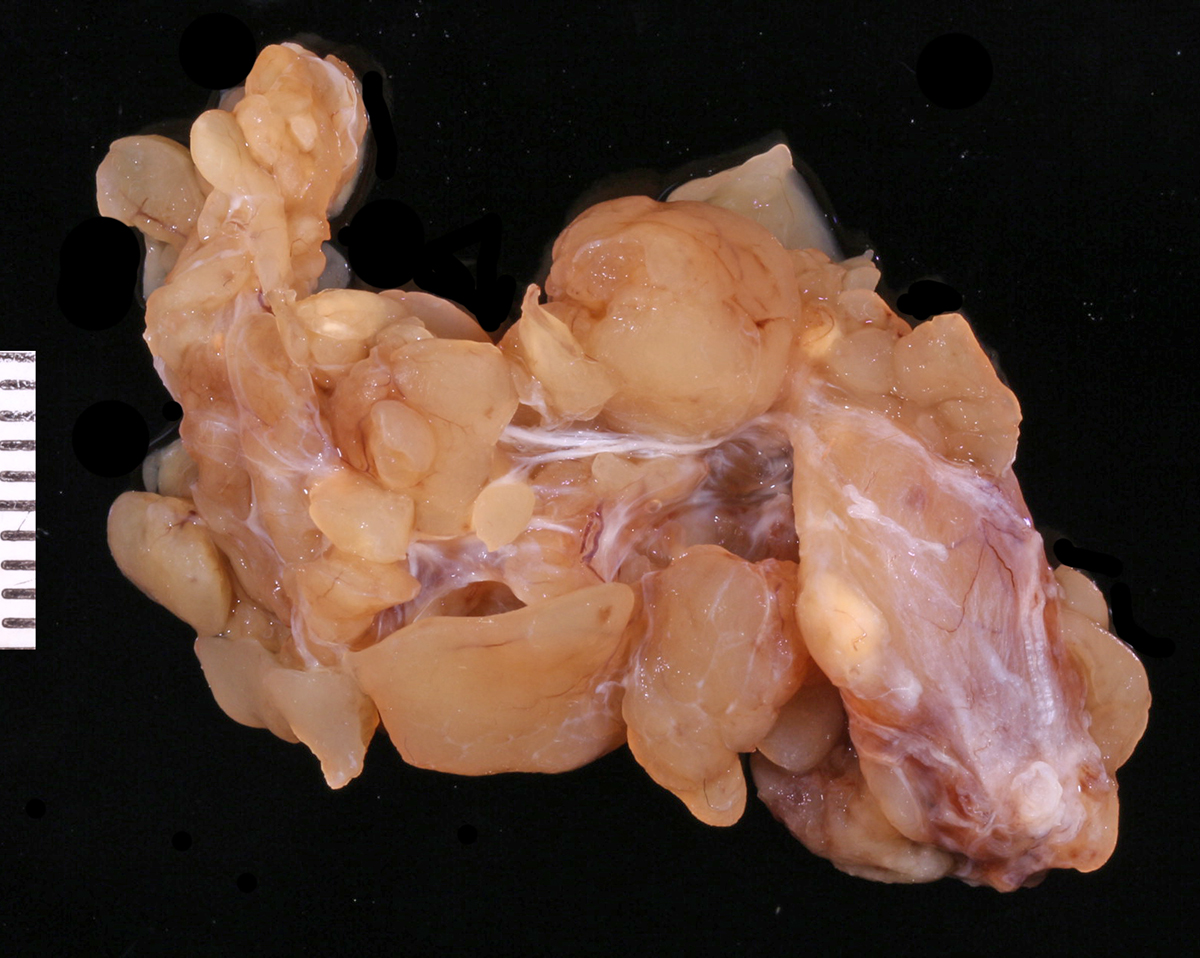
The submitted specimen consisted of multiple well demarcated and interconnected lobules of tan tissue and measured 3.5 x 1.8 x 1.0 cm. The consistency of the tissue was friable, with lobules falling away from the main mass during manipulation.
Laboratory Results:
Fine needle aspirate suggested an epithelial neoplasm.
Microscopic Description:
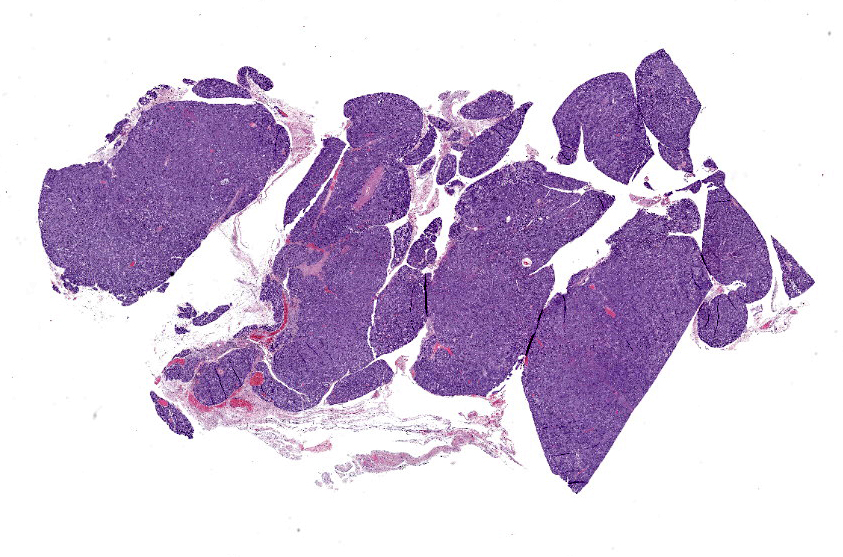
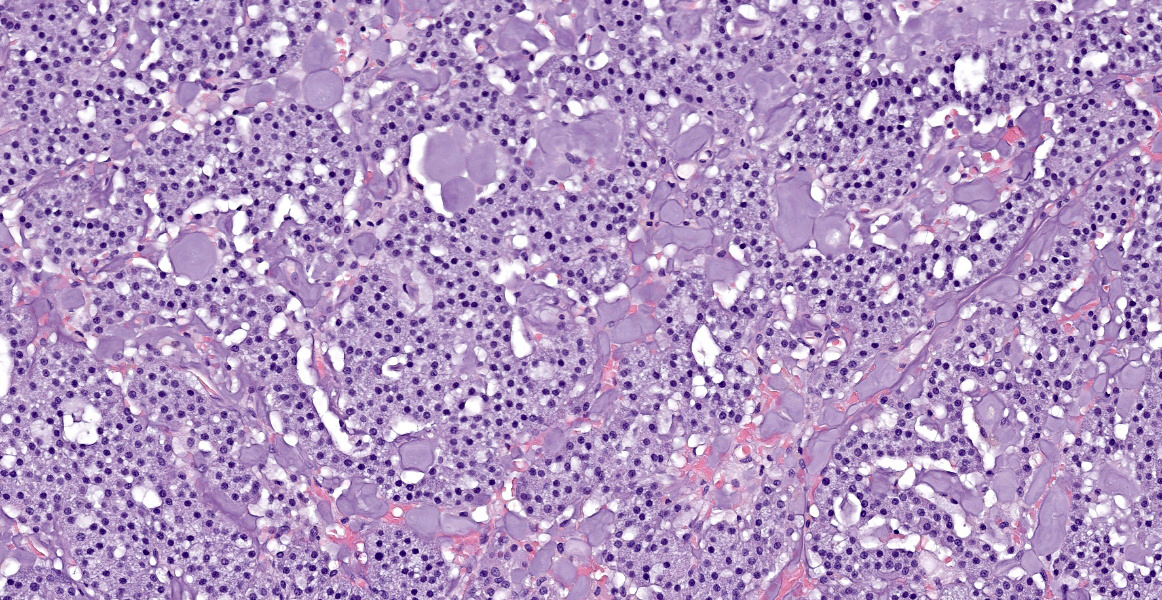
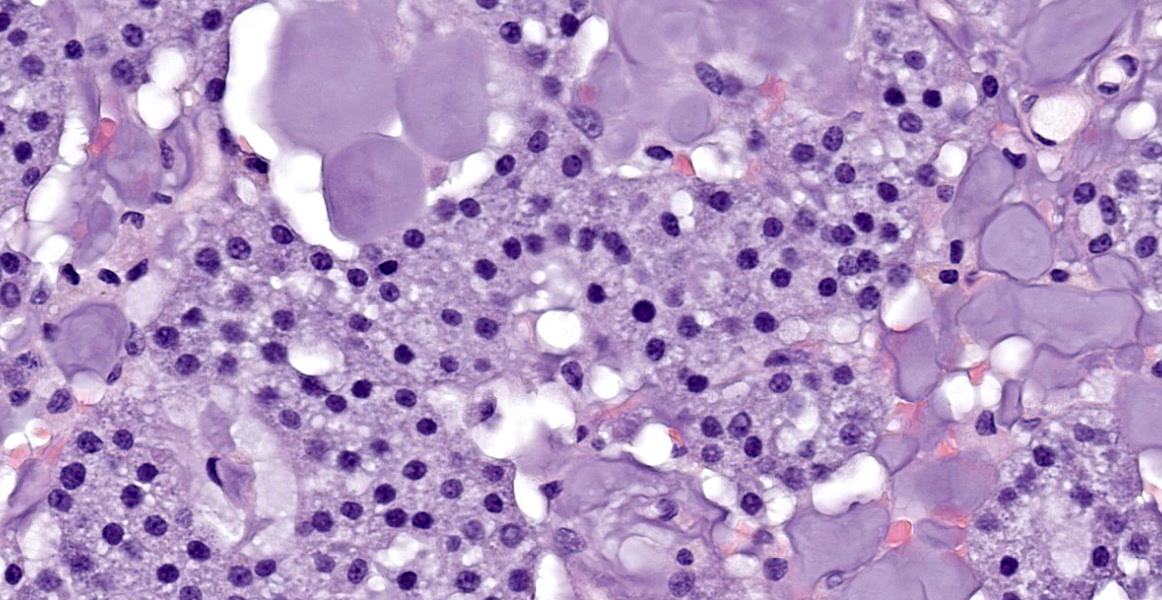
The tissue is mainly composed of an unencapsulated, poorly-delineated, densely cellular and multilobular neoplastic mass infiltrating and expanding the adjacent connective tissue. The mass is composed of cuboidal epithelial neoplastic cells, arranged into acini, cords and trabeculae supported by a delicate fibrovascular stroma. The neoplastic cells present moderate amounts of granular, lightly basophilic cytoplasm with indistinct cell borders, and round to oval nuclei with homogenous to condensed chromatin and 1-3 variably distinct nucleoli. Anisocytosis and anisokaryosis are mild, and no mitotic figures are seen. A homogenous basophilic secretory material is distributed between the trabeculae of neoplastic cells and sometimes throughout the extracellular space. There is a focal accumulation of foamy macrophages expandig the connective tissue adjacent to the mass. The neoplastic cells extend beyond the surgical margins.
Contributor’s Morphologic Diagnosis:
Orbital connective tissue: Canine lobular orbital adenoma.
Contributor’s Comment:
The gross and morphologic features of this mass are consistent with the diagnosis of canine lobular orbital adenoma. The key features attributed to this neoplasm include a well-differentiated glandular morphology, distinctive multi-lobular growth pattern, and a lack of glandular ducts and ductular differentiation of the neoplastic cells.2 These neoplasms are frequently described clinically and/or grossly as being remarkably friable, which is attributed to the scant amounts of connective tissue supporting the dense glandular lobular units. This neoplasm is associated with a high rate of local recurrence, which is attributed to its friable nature and tendency to disperse within the orbit, causing difficulties in achieving clean surgical margins.2 In the original case series describing this neoplasm, 10 out of 13 cases with available follow-up information experienced local recurrence (4 with multiple episodes of recurrence, with an average time after removal to recurrence of 395 days) with one case of recurrence associated with enucleation/exenteration, and the remaining 3 cases without recurrence being euthanized for reasons unrelated to this tumor.2 The origin of this neoplasm is unknown. Empirical data from the Comparative Ocular Pathology Laboratory of Wisconsin (COPLOW) suggests that accessory lacrimal glands observed
throughout the subconjunctival and orbital connective tissue are the most likely tissue of origin; however, lacrimal, third eyelid, or zygomatic salivary glands are other possibilities.2 Supporting evidence for a tissue of origin include location of the tumor within the orbit if it is relatively localized (e.g. supero-temporal suggesting lacrimal gland) and the morphologic and histochemical features of any adjacent non-neoplastic glandular tissue (e.g. primarily serous units suggesting lacrimal gland).1,2,6 The dorsal localization of this case’s neoplasm suggests it may be lacrimal in origin; however, this feature does not serve as definitive evidence. The examined tissues in this case did not include any non-neoplastic glandular tissue to support a specific tissue of origin.
Orbital neoplasia has been cited as the most commonly described disease of the orbit in dogs, with many of these neoplasms representing primary disease.2 However, metastatic tumors, neoplasia that extends into the orbit from nearby locations such as the oral and nasal cavities, and non-neoplastic orbital diseases such as orbital abscess also occur and must be differentiated.3
Contributing Institution:
University of Wisconsin
School of Veterinary Medicine
Madison, WI
https://www.vetmed.wisc.edu/departments/pathobiological-sciences/
JPC Diagnosis:
Periocular tissue: Canine orbital lobular adenoma.
JPC Comment:
Canine lobular orbital adenoma (CLOA) is a rather descriptive name for an enigmatic tumor. First formally described in a University of Wisconsin case series in 2004, little progress has been made in positively identifying the tissue of origin and descriptions of the entity often focus on distinguishing it from more well-known entities such as lacrimal or salivary gland tumors.2
Pathogenesis likewise remains mysterious. One recent study investigating the pathogenesis of this entity found no association between canine papillomavirus and the development of canine lobular orbital adenoma.4 Another recent study performed metagenomic analysis on 31 confirmed CLOAs looking for associations with an expanded group of DNA viruses in the neoplastic and normal conjunctival tissues and similarly found no associations.5
CLOAs occur in middle age to older dogs without breed predilection. The typical clinical presentation is a mass in the eyelid or subconjunctiva or, less commonly, in the retrobulbar tissues. Approximately 13% of cases are bilateral.5 Grossly, the neoplastic tissue appears nodular, translucent, and friable.
The histologic appearance of the tumor in this case is characteristic of CLOAs; there are abundant acini composed of epithelial cells filled with PAS-positive granules without accompanying ductal differentiation. Tissue samples submitted for histopathologic evaluation typically consist, as in this case, of neoplastic tissue without any surrounding orbital structures or contextual tissues, perhaps due to the neoplasm’s friable nature.2 When surrounding tissues are present for evaluation, the lobular growth characteristic of this neoplasm extends into the connective tissues of the orbit.2 The published immunohistochemical profile of CLOAs include positive immunoreactivity for CK 19 and AE1/AE3 and negative immunoreactivity for SMA, CK14, CALP, and p63, though this combination is not specific to this entity.6
Conference discussion focused mainly on the difficulty of tissue identification and histologic diagnosis. Conference participants offered a variety of differential diagnoses, including metastatic glandular tumors of the thyroid, lacrimal, sebaceous, and salivary glands, but even though diagnostically incorrect, all participants felt honored to be present for this novel tumor’s Wednesday Slide Conference debut.
References:
- Giudice C, Marco R, Mirko R, Luca M, Giorgio C. Zygomatic gland adenoma in a dog: histochemical and immunohistochemical evaluation. Vet Ophthalmol. 2005;8(1): 13-16.
- Headrick JF, Bentley E, Dubielzig RR. Canine lobular orbital adenoma: a report of 15 cases with distinctive features. Vet Ophthalmol. 2004;7(1):47-51.
- Hendrix DVH, Gelatt KN. Diagnosis, treatment and outcome of orbital neoplasia in dogs: a retrospective study of 44 cases. J Small Anim Pract. 2000;41(3):105-108.
- Schaefer EAF, Chu S, Pearce JW, Bryan JN, Flesner BK. Papillomavirus DNA not detected in canine lobular orbital adenoma and normal conjunctival tissue. BMC Vet Res. 2019;15:226.
- Schaefer EAF, Chu S, Wylie KM, et al. Metagenomic analysis of DNA viruses with targeted sequence capture of canine lobular orbital adenomas and normal conjunctiva. Microorganisms. 2023;11(5):1163.
- Wang F, Ting CT, Liu Y. Orbital adenocarcinoma of lacrimal gland origin in a dog. J Vet Diagn Invest. 2001;13(2):159-161.