Signalment:
Gross Description:
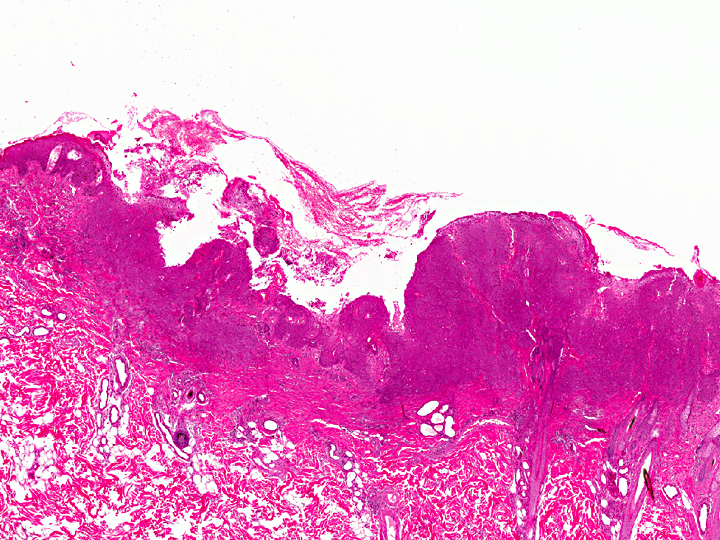
Histopathologic Description:
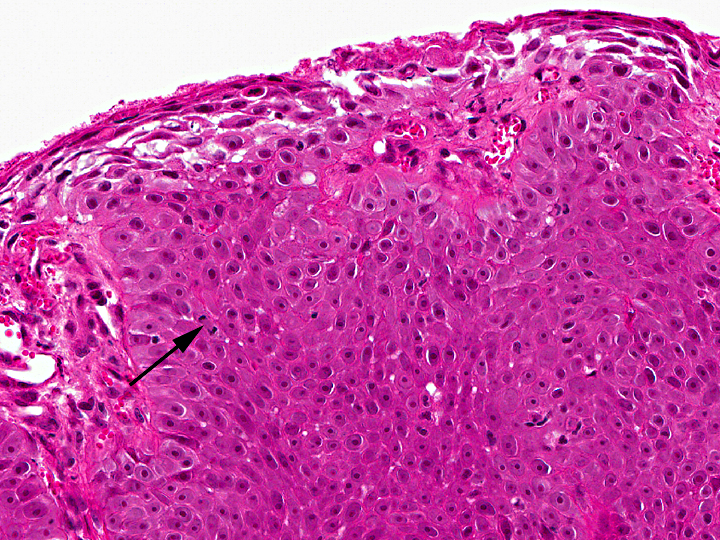
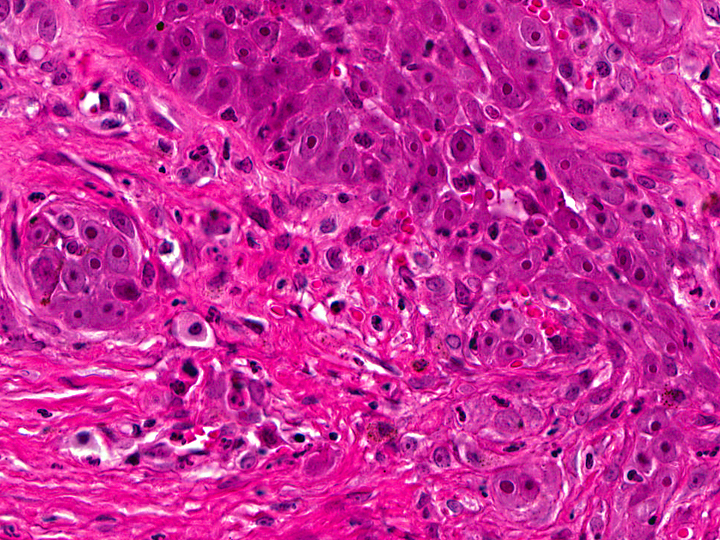
In the thickened epithelial areas the normal germinal layer of the epidermis is replaced by multiple layers of neoplastic keratinocytes. The stratum granulosum and stratum corneum are mostly unaffected. Neoplastic cells have an increased size (about 30-50 μm in diameter), a polygonal shape and a small to moderate amount of homogenous to finely granular eosinophilic cytoplasm. Distinct cell borders and prominent intercellular bridging are present. Nuclei are large, round to oval, centrally placed, and vesicular with finely stippled chromatin and one to two prominent round magenta nucleoli. There is a mild to moderate anisocytosis and anisokaryosis. The number of mitotic figures range from 0 to 4 per high power field. Multifocally, dark basophilic round structures are present distributed within the neoplastic cells, interpreted as apoptotic bodies. In some areas the above described neoplastic cells penetrate the basement membrane and invade the underlying dermis, forming small nests or cords. There is multifocal erosion and focal ulceration of the epidermis, associated with hemorrhage and mild to moderate infiltration of neutrophils. In the dermis a mild, perivascular infiltration of neutrophils, lymphocytes and macrophages is present and few mast cells are observed. Multifocally there is moderate fibrosis of the dermis.
No p53-positive tumor cells were detected by immunohistochemistry using a monoclonal anti-p53-antibody.
Morphologic Diagnosis:
Condition:
Contributor Comment:
Grossly, irregular, slightly elevated to heavily-crusted plaques and verrucous or papillary lesions up to 5.0 cm in diameter are found on haired, pigmented or non-pigmented skin at any site of the body. Usually, multiple lesions occur, but also solitary MSCCa in situ are seen infrequently.(1, 6, 8) Lesions are reported to be chronic, not painful, and only mild pruritus is noted.(1) There is neither a casual relationship to sunlight exposure nor a breed or sex predilection.(6, 7, 8)
Histologically, the neoplastic keratinocytes are confined to the epidermal and follicular infundibular epithelium with the basement membrane remaining intact.(1, 6) Some authors differentiate between an irregular nonhyperkeratotic and a verrucous hyperkeratotic type depending on the character and degree of epidermal thickening and hyperkeratosis.(1) According to this classficiation, the present case would belong to the non-hyperkeratotic type due to severe acanthosis of the epidermis and follicular infundibular epithelium with a mildly undulating surface and mild orthokeratotic hyperkeratosis, not showing verrucous surface contours, epithelial spires and pit-like invaginations filled with keratin as it is described for the verrucous hyperkeratotic type.(1)
Several studies suspect that papillomavirus infection may be associated with feline MSCCa in situ. In two studies, papillomavirus-antigen was detected in 11% and 48% of MSCCa in situ, respectively, using immunohistochemistry (IHC).(2, 13) Additionally, it has been hypothesized that feline viral plaque, in which a high proportion of papillomavirus-antigen was detected by immunostaining, could be a precursor lesion of MSCCa in situ.(16)
One study found 11 out of 18 cases positive for papillomavirus L1 gene in PCR-analysis, suggesting that this method may be more sensitive than IHC.(11) The authors admit that the failure to demonstrate papillomaviral DNA in every lesion may suggest that MSCCa in situ is not caused by papillomavirus infection. However, they also state that the 7 negative cases may have been previously but transiently infected with papillomavirus, which may have caused cellular transformation.(11) Furthermore, a close relationship to human papillomaviruses was discovered, which led to the hypothesis of interspecies virus transmission.(11) Subsequently, multiple papillomaviruses (namely Felis domestics-papillomavirus type 1, type 2 and a novel papillomavirus) from a swab of feline viral plaques and non-lesional skin were amplified, suggesting that papillomavirus can infect the epidermis without causing appreciable disease as well.(12)
In one report, infestation with Demodex cati, a normal resident of the skin in cats, was found in lesional sites of MSCCa in situ in 4 cats. Local cutaneous immunodeficiency due to epithelial dysplasia has been hypothesized as a predisposing factor for focal multiplication of mites.(9) In some cases, it is proposed thatimmunosuppression due to FIV or FELV infection may be a possible predisposition.(9, 12)
For differential diagnosis, difficulty in distinguishing between the two basic types of squamous cell carcinoma in situ in cats have to be considered: MSCCa in situ and actinic keratosis (also named solar keratosis).(2) Actinic keratosis usually occurs as solitary lesion in white animals or in locations of unpigmented skin (like pinna, planum nasale, dorsal muzzle and eyelids).(2) Its histological appearance is less hyperplastic and hair follicles are less deeply affected than in MSCCa in situ.(2) Nevertheless, an accurate classification by clinical and histological findings is sometimes difficult.(2, 8) Therefore, immunohistochemical detection of papillomavirus-antigen, which could be indicative of MSCCa in situ, and of p53-protein, which is frequently accumulated after UV-induced mutation of the p53 gene in actinic keratosis could be performed to arrive at the diagnosis.(2)
Similar to the present case, 17% to 25% of feline cases MSCCa in situ become locally invasive through the basement membrane and then have to be classified as squamous cell carcinomas, the most common malignant neoplasm of the feline skin.(1, 7, 8) These lesions, belonging to the well differentiated squamous cell carcinomas, occasionally still show bowenoid features like dorsoventrally elongated nuclei, that are tilted in one direction (windblown pattern) or bizarre multilobulated nuclei with smudgy chromatin.(8)
It has to be kept in mind that the described squamous cell carcinoma may also have developed due to UV-light induced mutations of tumor suppressor genes, unrelated to the diagnosed MSCCa in situ.(8)
Other skin tumors should be considered as additional differential diagnoses. Basal cell carcinomas and basosquamous carcinomas, which arise from basal cells, are usually located in the dermis. However, they can be associated with the epidermis and are defined by other characteristics: in basal cell carcinomas, extensive proliferation of stromal fibroblasts and horizontal orientation of the tumor silhouette are frequently found. The appearance of bimorphic histologic features, showing basaloid cells peripherally and centrally abrupt keratinisation is indicative for basosquamous carcinomas.(7, 8)
JPC Diagnosis:
1. Haired skin: Squamous cell carcinoma.
2. Haired skin: Bowenoid in situ carcinoma.
Conference Comment:
SCC is typically locally invasive and slow to metastasize; however, SCCs arising from internal sites such as the tonsil, stomach, and urinary bladder are more prone to metastasize, and metastasis is often to the lungs and regional lymph nodes. In cattle, SCC is common on eyelids, conjunctiva, and the vulva; in horses, it is common on the penis, prepuce, eyelid and conjunctiva, and is also found in the nonglandular stomach; in rodents and pigs, SCC is also common in the nonglandular stomach; in sheep and goats, the vulva in recently sheared sheep is a common site as well as the pinnae; in rabbits it is found in subungual tissues, the eyelid and extremities; and SCC can also occur at brand sites (especially freeze brands) in livestock (3, 4, 5, 14, 15).
References:
2. Favrot C, Welle M, Heimann M, Godson DL, Guscetti F. Clinical, histologic and immunohistochemical analyses of feline squamous cell carcinoma in situ. Vet Pathol. 2009; 46:25-33.
3. Foster RA. Female reproductive system and mammary gland. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 4th ed. vol. 1 St. Louis, MO: Elsevier; 2012:1078, 1118.
4. Foster RA. Male reproductive system. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 4th ed. vol. 1 St. Louis, MO: Elsevier; 2012:1149, 1178.
5. Gelberg HB. Alimentary system and the peritoneum, omentum, mesentery, and peritoneal cavity. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 4th ed. vol. 1 St. Louis, MO: Elsevier; 2012:328-9, 354.
6. Ginn PE, Mansell JEKL, Rakich PM. Skin and appendages. In: Maxie MG, ed. Jubb, Kennedy, and Palmer-�s Pathology of Domestic Animals. 5th ed. Edinburgh, UK: Elsevier; 2007: 752-753
7. Goldschmidt MH, Dunstan RW, Stannard AA, von Tscharner C, Walder EJ, Yager JA. Histological classification of epithelial and melanocytic tumors of the skin in domestic animals. Washington DC: Armed Forces Institute of Pathology; 1998: 18-21
8. Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Skin diseases of the dog and cat: clinical and histopathological diagnosis, 2nd ed. Oxford, UK: Blackwell science; 2005: 148-151, 575-581, 583-584, 591-594, 597-598
9. Guagu+�-�re E, Olivry T, Deleverdier-Poujade A, Denerolle P, Pag+�-�s JP, Magnol JP. Demodex cati infestion in association with feline cutaneous squamous cell carcinoma in situ: a report of five cases. Vet Dermatol. 1999; 10: 61-67.
10. Hargis AM, Ginn PE. The integument. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 4th ed. vol. 1 St. Louis, MO: Elsevier; 2012:1013-27.
11. Munday JS, Kiupel M, French AF, Howe L, Squires RA. Detection of papillomaviral sequences in feline Bowenoid in situ carcinoma using consensus primers. Vet Dermatol. 2007;18:241-245.
12. Munday JS, Willis KA, Kiupel M, Hill FI, Dunowska M. Amplification of three different papillomaviral DNASequences from a cat with viral plaques. Vet Dermatol. 2008; 19: 400-404.
13. Munday JS, Kiupel M. Papillomavirus-associated cutaneous neoplasia in mammals. Vet Pathol., 2010; 47: 254-264.
14. Newman SJ. The urinary system. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 4th ed. vol. 1 St. Louis, MO: Elsevier; 2012:648.
15. Njaa BL, Wilcock BP. The ear and eye. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 4th ed. vol. 1 St. Louis, MO: Elsevier; 2012:1237-8.
16. Wilhelm S, Degorce-Rubiales F, Godson Dale, Favrot C: Clinical, histological and immunohistochemical study of feline viral plaques and bowenoid in situ carcinomas. Vet Dermatol. 2006; 17: 424-431.
17. Yager JA, Wilcock BP. 16 Solid epidermal tumors (papilloma, cutaneous horn, keratoses, squamous cell carcinoma). In: Color Atlas and Text of Surgical Pathology of the Dog and Cat. London, UK: Wolfe Publishing; 1994: 253-254