CASE 2: No label (4135756-00)
Signalment:
18-year-old, gelding, Friesian horse, Equus caballus
History:
Depressed/dull mentation for the last 4 days; progressive neurologic signs, head pressing, weakness, falling down. Has had progressive weight loss over the winter.
Gross Pathology:
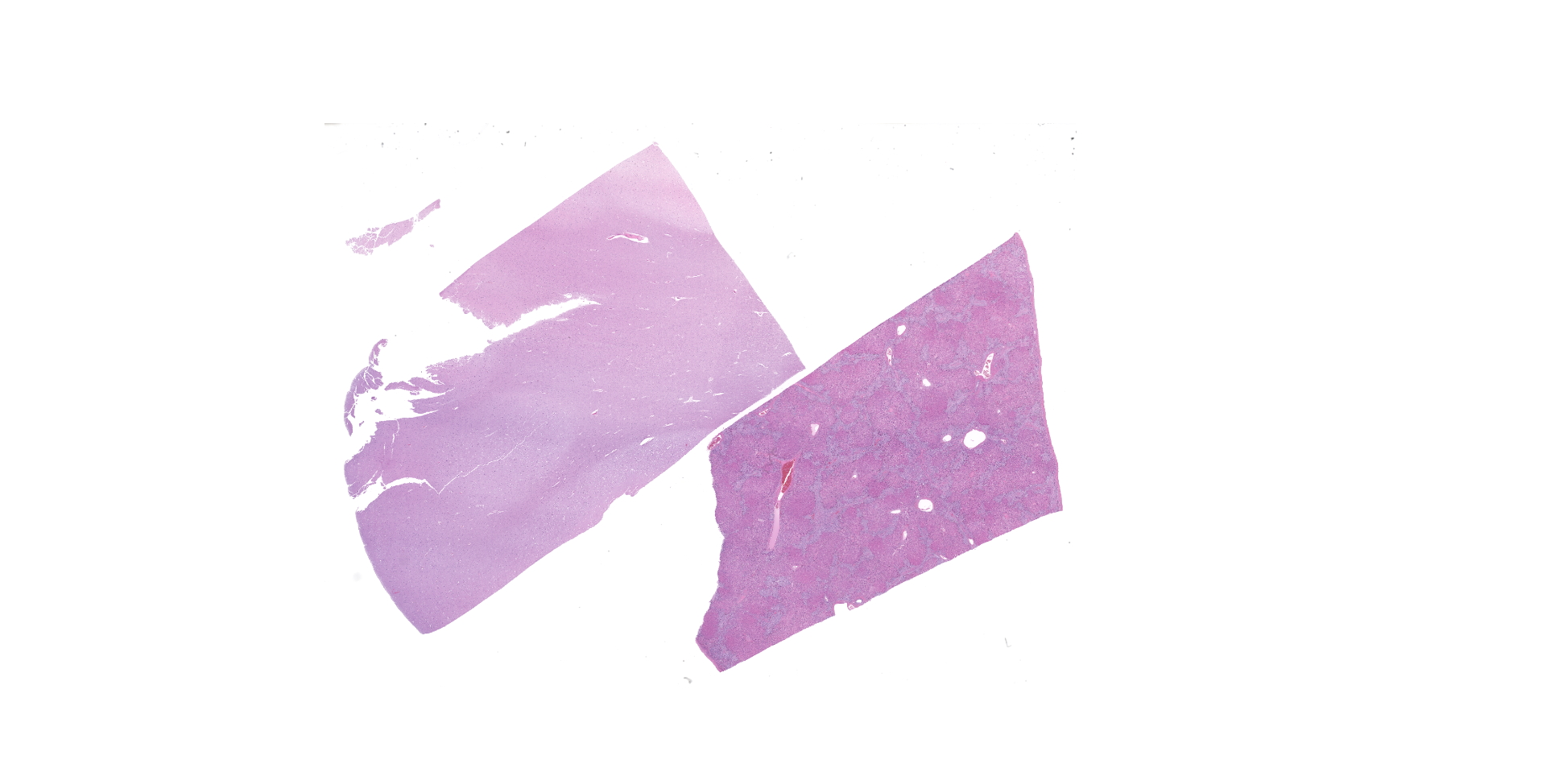
A complete gross necropsy was performed. Notable gross findings were limited to the liver, which was diffusely pale green to tan with a subtle reticular pattern that continued on cut surface. Diffusely the liver was markedly firm and contracted. No gross brain lesions were identified.
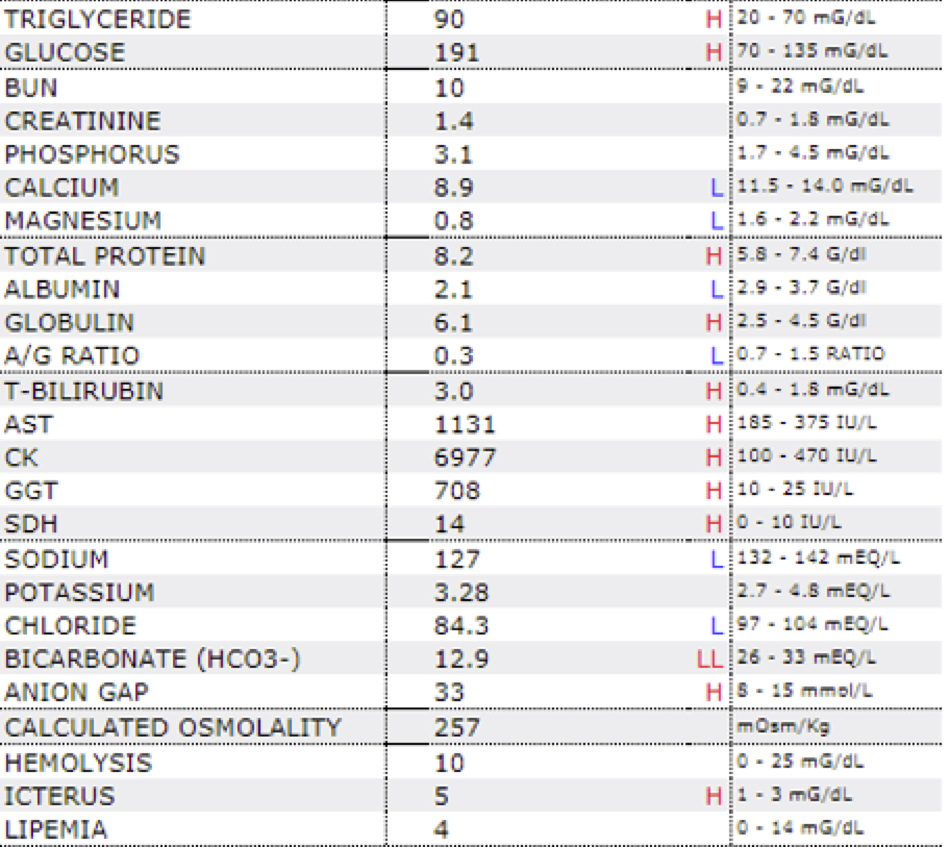
Laboratory results:
See table.
Microscopic description:
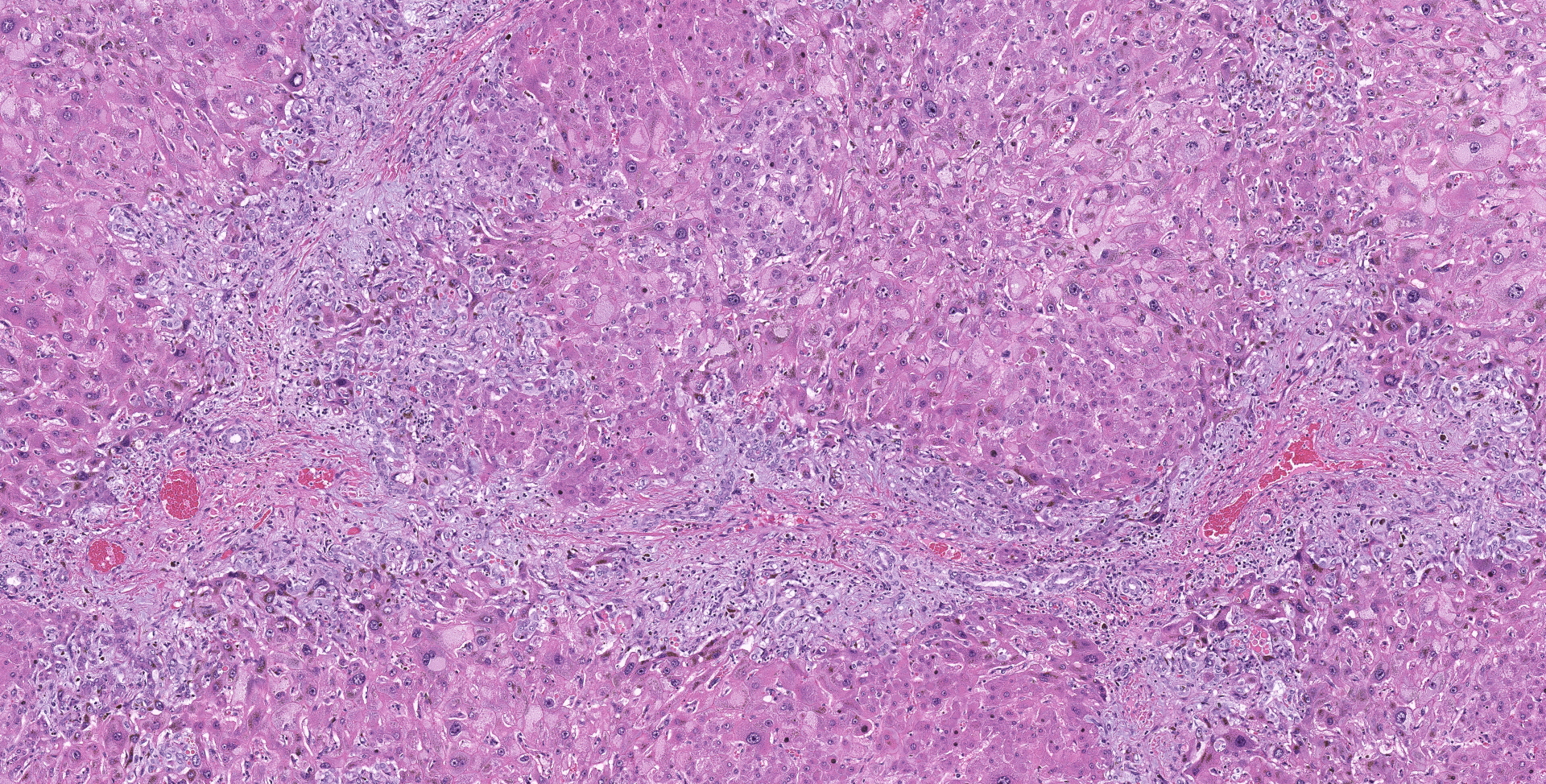
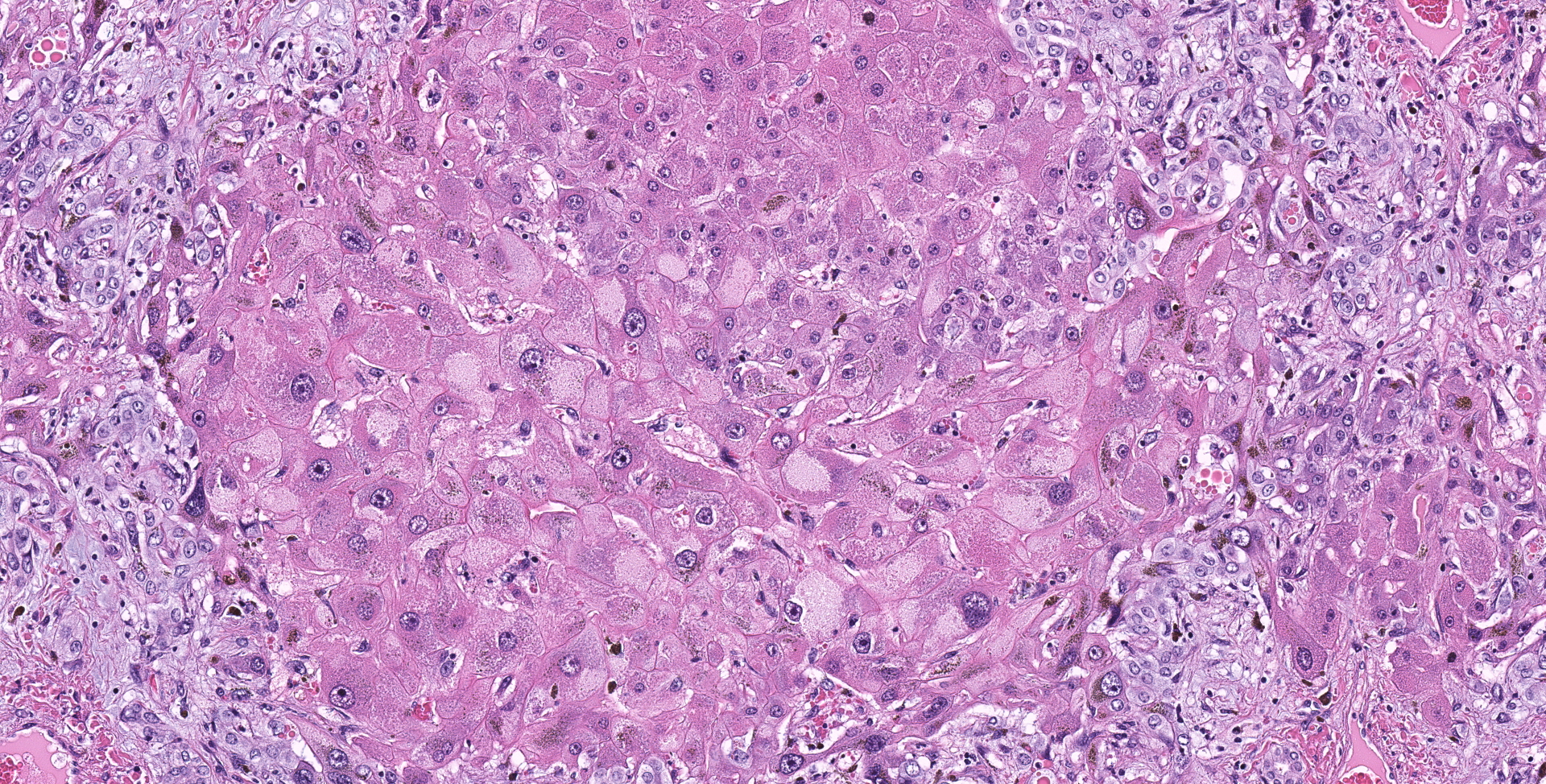
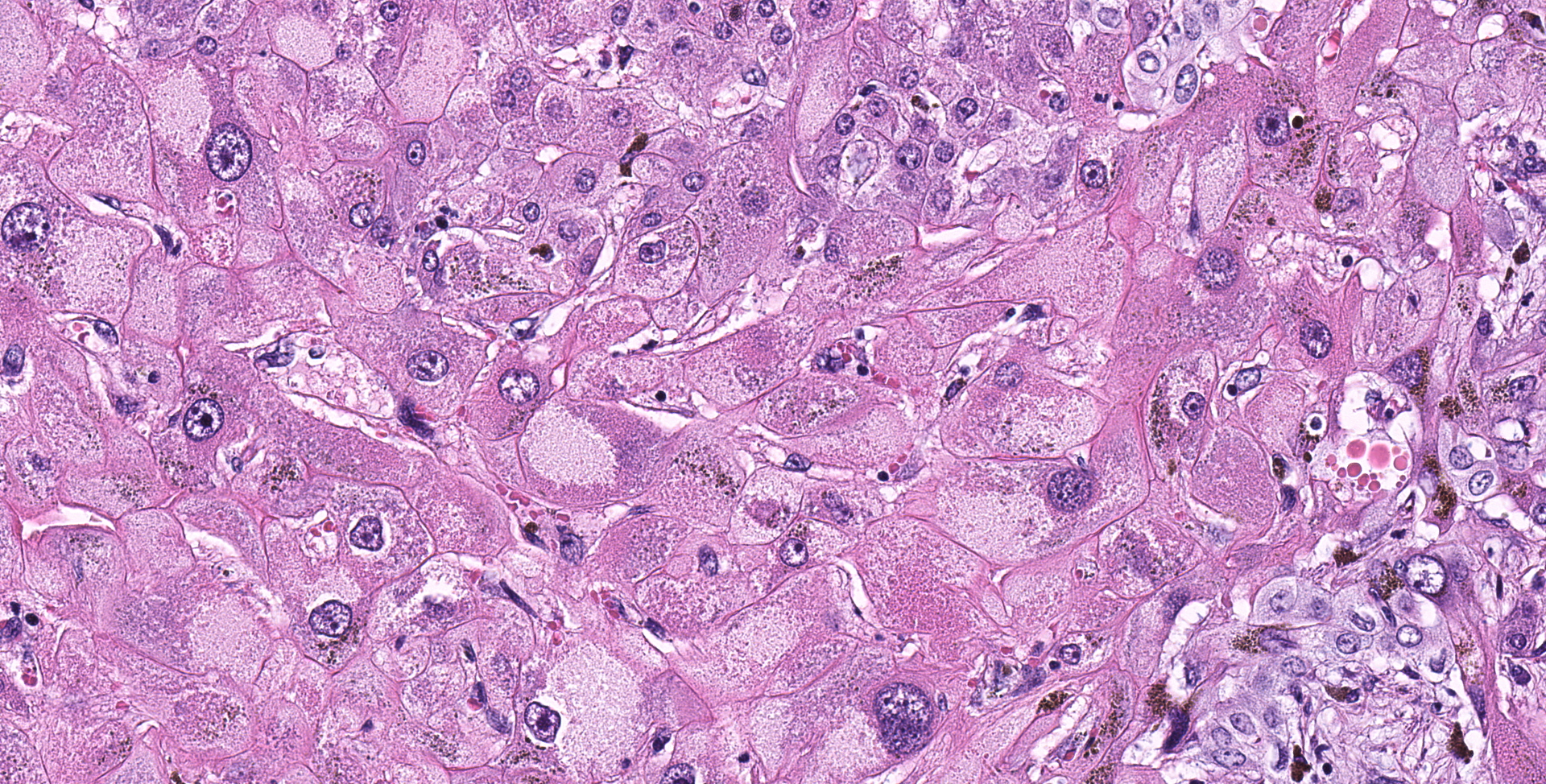
Liver: Diffusely hepatocytes are degenerative and dysplastic with marked variation in cellular morphology characterized by abundant, variable vacuolated, cytoplasm (cytomegaly) and marked nuclear variation with stippled chromatin and prominent nucleoli (karyomegaly). Hepatocytes occasionally have two to several nuclei. Rarely affected hepatocytes are necrotic with shrunken cellular profiles, hypereosinophilic cytoplasm, cellular rounding and pyknotic to karyorrhectic nuclei. The interstitium is infiltrated by low numbers of individualized lymphocytes, plasma cells and pigment laden macrophages. Portal triads are diffusely expanded by an abundant amount of fibroplasia that dissects into the surrounding parenchyma and commonly connects adjacent portal triads (bridging fibrosis). Portal triads also have increased numbers of biliary profiles (biliary hyperplasia) and a moderate amount of oval cell hyperplasia. Multifocally hepatic lobules are hypercellular and expanded with loss of discrete architecture and portal triad drop out (nodular regeneration).
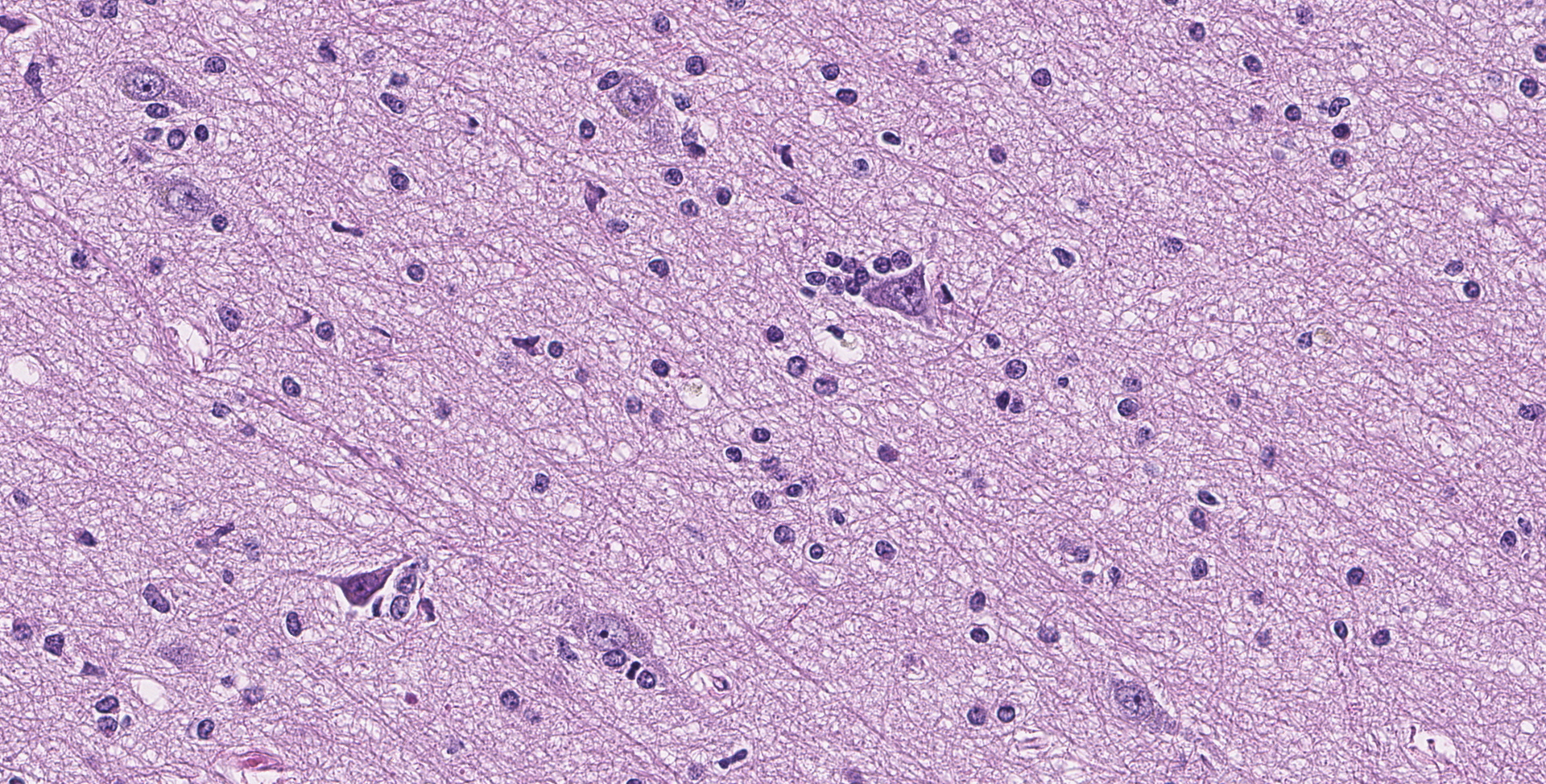
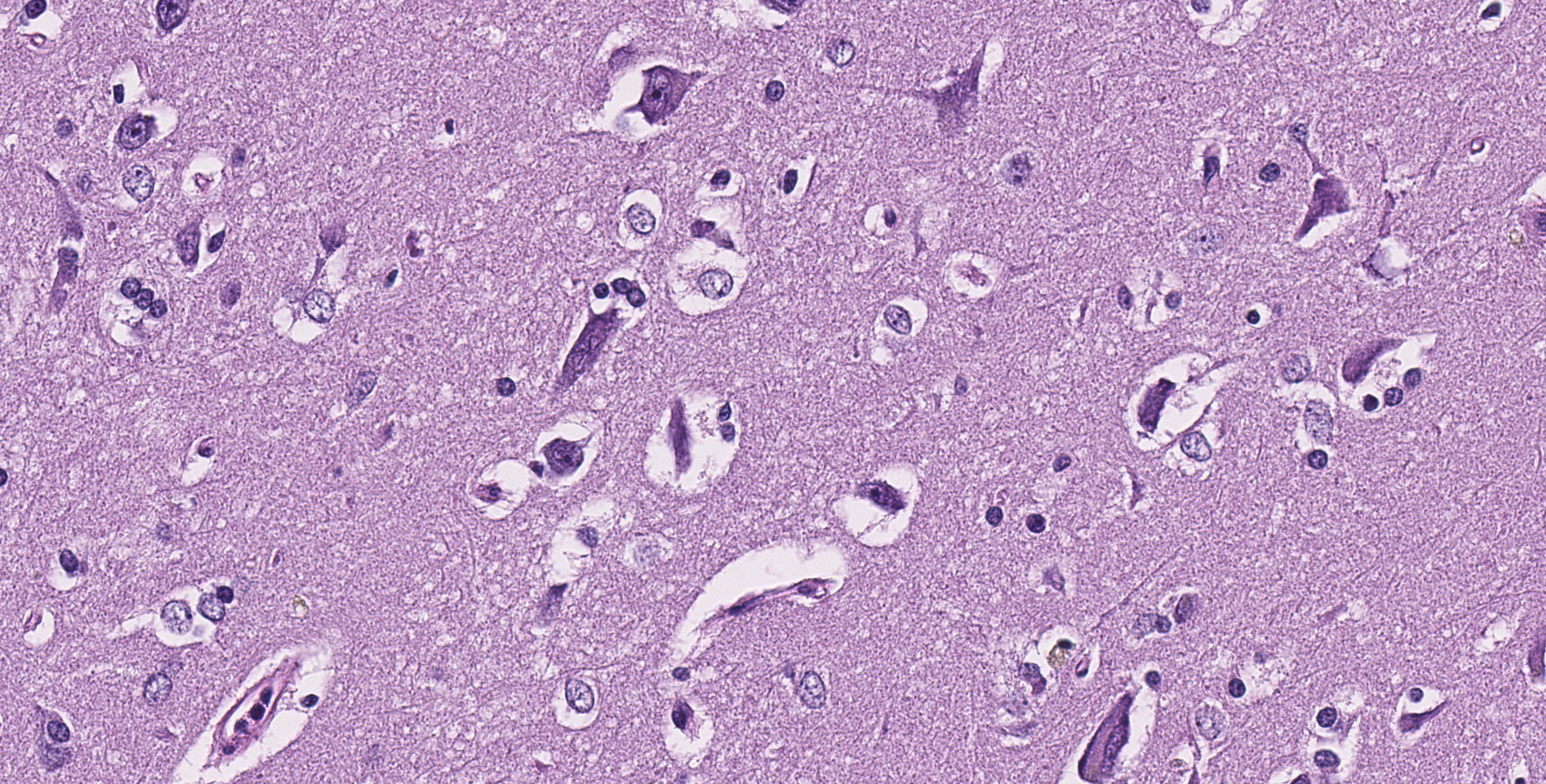
Cerebrum: Throughout the gray matter are an increased number of astrocytes with many pairs and clusters of astrocytes with enlarged vesicular nuclei that are multifocally surrounded by a non-staining rim (type 2 Alzheimer cells). Rarely, neurons in affected regions are hypereosinophilic and angular (necrotic). Small and medium caliber vessels have expanded Virchow-Robin spaces (vasogenic edema).
Contributor's morphologic diagnosis:
Liver: Marked, chronic, toxic hepatocellular degeneration with portal and bridging fibrosis, biliary hyperplasia, megalocytosis and megalokaryosis, rare individual hepatocyte necrosis and nodular regeneration.
Brain: Moderate, chronic, diffuse Alzheimer type 2 astrocytosis with multifocal neuronal necrosis and degeneration.
Contributor's comment:
The triad of changes appreciated in the liver, namely the fibrosis, biliary hyperplasia, and megalocytosis with karyomegaly, are nearly pathognomonic for pyrrolizidine alkaloid (PA) toxicosis in horses. PAs are found within many different plants, particularly in the Borraginaceae, Compositae and Leguminosae families.1 PA is thought to be produced by plants to dissuade foraging by grazing animals. Many animals will not choose to eat PA containing plants unless better forage is not available. Several plant species are particularly problematic for domestic species, which includes Senecio, Crotalaria, Heliotropium, Amsinckia, Echium, and Cynoglossum species.3,5 In this particular case the exact plant species was not identified.
PA is an indirect hepatotoxin requiring phase 1 biotransformation from zone 3 hepatocytes utilizing P450 cytochrome (mainly CYP3A and CPY2B6).7 Three common pathways of PA metabolism have been identified including hydrolysis of PAs to release necines and necic acid, N-oxidation to form PA N-oxides, and oxidation of PAs to produce dehydro-pyrrolizidine (pyrrolic ester) derivatives.2,7 The two former pathways are considered pathways of detoxification that lead to excretion of the PA via the urinary system. This is thought to partially determine differences in species susceptibility with rats lacking the required esterase activity and thus being very susceptible, while guinea pigs are highly resistant to toxicity.2 Biotransformation via the third pathway of PA forms the dehydropyrrolizidine alkaloid (dPA). DPA is extremely reactive which can covalently bind to DNA or protein forming DNA adducts, protein adducts and DNA and protein cross links.7
The cumulative effects of the toxic products lead to DNA replication but inhibition of successful mitosis. This excessive DNA replication without cell division leads to the microscopically appreciated cytomegaly and karyomegaly.1,3,5 However, hepatocytes are not uniformly affected with some cells retaining the ability to successfully replicate. This leads to the gross and histologic manifestation of small nodules of more typical regenerative hepatocytes. The corresponding biliary hyperplasia, typically prominent in PA toxicity, is thought to be a response to the local growth factors and stimuli released with chronic-active hepatocyte injury. Fibroplasia in these cases can be extremely varied with some species-specific findings with sheep having minimal fibroplasia and cattle displaying marked fibrosis.
Three clinical forms of PA toxicity have been well established and include acute, phasic and prolonged exposure. The acute form is rare, as animals generally avoid ingesting large quantities of plants that contain high levels of PA due to poor palatability. This forms usually occurs with cases of starvation (when other forage is not available) or with experimental induction. It manifests with marked acute centrilobular to submassive necrosis with hemorrhage. The second, and most common form, is phasic or seasonal exposure. This form occurs with seasonal low-level grazing of plants with PA and manifests, as demonstrated in this case, with megalocytosis, biliary hyperplasia, and fibroplasia. The third form is prolonged exposure and is almost exclusively induced by Heliotropium, usually in sheep. This form leads to a small markedly fibrotic liver, which lacks the characteristic regenerative hepatocytes or megalocytosis.3,5
As mentioned previously, there is a noticeable variation in presentation amongst species, which is multifactorial. Ruminants are considered more resistant than monogastrics due to the degradation of the PA within the ruminal fermentation processes. Amongst ruminants, sheep are considered to be the most resistant, requiring 20 times the concentration by oral exposure when compared to cattle.3 When affected, sheep classically present with the prolonged form of toxicosis due to Heliotropium. Pigs are considered to be the most susceptible species, while horses are moderately susceptible and present with either the acute or more phasic forms.1,3,5
Although the liver is commonly considered the primary tissue affected by PA toxicity other tissues can be injured, some of which have certain plant specificities. It has been demonstrated that there is injury to endothelial cells within the liver, leading to the large degree of hemorrhage appreciated within the acute form. The injury here has been speculated to be from leakages of reactive PA from adjacent hepatocytes.2,3 Some PA containing plants, such as Crotalaria, have a wider tissue effect, commonly affecting renal convoluted tubules and Clara cells within the lungs. These tissue toxicities are thought to be due to the biotransformative properties of these cells.
As mentioned, horses have intermediate susceptibility to PA toxicity, but have a high chance of developing hepatic encephalopathy in the face of a toxic insult, as demonstrated in this case. Hepatic encephalopathy in due to the increased concentration of ammonia and other liver related metabolites. Ammonia is considered directly neurotoxic, with astrocytes serving as an important cell for ammonia detoxification. Astrocytes detoxify ammonia via the synthesis of glutamine via glutamine synthase. Increased levels of glutamine in astrocytes cause astrocyte swelling, forming the classic type 2 astrocytes.8 Interestingly, horses with hepatic encephalopathy don't present with spongiform change at the white and gray matter junction. The exact pathogenesis of spongiform change, and why horses don't have this manifestation, have not been clarified.
Contributing Institution:
Colorado State University
http://csu-cvmbs.colostate.edu/vdl/Pages/default.aspx
JPC diagnosis:
Liver: Fibrosis, portal and bridging, diffuse, moderate, with hepatocellular loss, megalokaryosis, megalocytosis, cholestasis, and ductular reaction (biliary hyperplasia), Friesian, equine.
Cerebrum: Alzheimer's type 2 astrocytosis, diffuse, moderate, with gliosis, neuronal necrosis and satellitosis.
JPC comment:
During conference discussion, there was lively debate surrounding the severity of degenerative neuronal changes, which was largely a result of slide variation among participants.
While pyrrolizidine alkaloid (PA) compounds cause harm to veterinary species, they also have detrimental effects in humans. While animals often graze at the margins of pasture, or have PA containing plants co-harvested with feed plants, human PA toxicity is often more direct. In some areas of the world, dehydropyrrolizidine alkaloid (DHPA) producing plants have been eaten or used as part of herbal medicine for generations. There are a number of recent herbal products and remedies that contain DHPA compounds and are not regulated in any way (similar to nutrition supplements in the United States). Thousands of people were poisoned in Afghanistan and Tajikistan when grain, and subsequently grain, was contaminated. However, the scope of the problem is enormous, with more than 3000 plant species used in more than 50,000 herbal products, as of 2016. A number of government health organizations and agencies have developed regulations and recommendations for products containing DHPA compounds, including the United States, United Kingdom, Germany, European Food Safety Authority, Dutch National Institute for Public Health, World Health Organization, and Food Standards Australia New Zealand.6
Based on the reactions required to metabolize DHPA compounds, it is reasonable to ponder whether they have carcinogenic or genotoxic effects. Testing individual DHPA compounds is difficult, because plants often contain only 2-3% of a compound and may be part of numerous toxic compounds. However, research has been conducted on riddelliine in rats and mice, because Senecio riddellii contained approximately 18% riddelliine and nearly no other DHPA compounds. Multiple murine studies have found dose dependent increases in the rates of neoplasia in subjects, with the most common finding hepatic hemangiosarcoma. A study using p53 knockout mice replicated those findings and found that even exposure at the lowest dose (5 mg/kg/day) for only 14 days resulted in an increased incidence of neoplasia. While evidence is incomplete, it is reasonable to assume other untested DHPA compounds may produce similar effects.6
Donkeys have recently been used to research two species of Crotalaria and determine if they affect the same tissues in a similar manner. However, in the tested donkeys, Crotalaria retusa exposure was only associated with liver lesions at either the low or high dose, while Crotalaria juncea exposure primarily affected the lungs at high doses. Because the primary DHPA compounds in the two plants are different, this result suggests either that a toxic DHPA metabolite produced in the liver is released to systemic circulation and causes damage in the lung, or that the whole DHPA compound avoids metabolism in the liver and is oxidized by cytochrome P450 systems of club cells in the lung. In the study donkeys, because there were few liver lesions, it may be more likely that the majority of the DHPA compounds bypass the liver and are metabolized in the lung.4
References:
1. Brown DL, Wettere AJV, Cullen JM, Hepatobiliary system and exocrine pancreas. in Zachary JF. Pathologic Basis of Veterinary Disease. St. Louis, MO: Mosby; 2017: 449-450
2. Chen, T., Mei, N., & Fu, P. P. (2010). Genotoxicity of pyrrolizidine alkaloids. Journal of Applied Toxicology. doi:10.1002/jat.1504
3. Cullen JM, Stalker MJ, Liver and Biliary system. in: Jubb KV, Kennedy PC, & Palmer N. Pathology of domestic animals. San Diego, CA: Academic Press; 2015: 337-338
4. Pessoa CRM, Pessoa AFA, Maia LA, et al. Pulmonary and hepatic lesions caused by the dehydropyrrolizidine alkaloid-producing plants Crotalaria juncea and Crotalaria retusa in donkeys. Toxicon. 2013;71:113-120.
5. Stalker MJ, Hayes MA. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. Vol 2. 5th ed. Philadelphia, PA: Elsevier; 2007:373-374
6. Stegelmeier BL, Colegate SM, Brown AW. Dehydropyrrolizidine Alkaloid Toxicity, Cytotoxicity, and Carcinogenicity. Toxins (Basel). 2016;8(12):356. Published 2016 Nov 29. doi:10.3390/toxins8120356
7. Wiedenfeld, H., & Edgar, J. (2010). Toxicity of pyrrolizidine alkaloids to humans and ruminants. Phytochemistry Reviews,10(1), 137-151. doi:10.1007/s11101-010-9174-0
8. Zachary JF: Nervous system. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Mosby; 2007:814-815