WSC 2022-2023
Conference 13
Case 1
Signalment:
Two, 8 month old female entire Boer goats (Capra hircus)
History:
A herd of nine goats were kept with a flock of sheep in a paddock associated with a school agricultural program. The goats gained access to an adjacent paddock of shrubbed land through a hole in the fence which the sheep did not find, and had been foraging in this adjacent paddock for approximately one week. Two goats were found dead initially, and a further two became acutely lethargic and depressed, and died early the following day (these two were submitted for necropsy). Within 24 hours, a further three goats had died, leaving one remaining goat from the herd that was also exhibiting acute clinical signs. One dead goat was found underneath a Trema tomentosa (poison peach) tree, and a further two that were observed beneath the Trema tomentosa subsequently died. A video was submitted of an affected goat, which showed a standing goat with a wide-based stance, salivation/foam around the mouth, and twitching of the upper lip and eyelids, with apparent unawareness of the surroundings.
Plants within the shrubbed area accessed by the goats included Trema tomentosa (Poison peach), Acacia fimbriata (Brisbane wattle), Solanum mauritianum (Wild tobacco), Schinus terebinthifolius (Broad-leaf pepperina), and an unknown species of wattle, (Acacia sp.) as identified by a botanist with the Queensland Herbarium.
Gross Pathology:
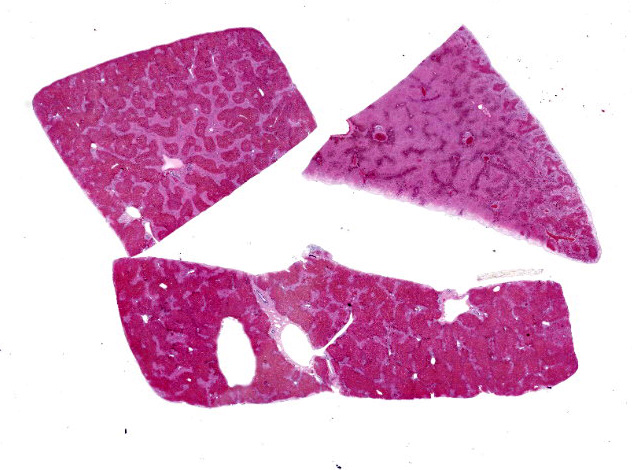
In one goat, the liver had a generalized, exaggerated reticular pattern, interpreted as periacinar necrosis. The same goat also had a mild pleural effusion and petechial hemorrhages in the subcutaneous tissues of the ventral neck and serosal surfaces of the rumen and small intestine. The second goat appeared slightly more autolyzed than the first, and had no grossly observed pathology. The rumen of both goats contained fragments of foliage from multiple plants species.
Laboratory Results:
No findings reported.
Microscopic Description:
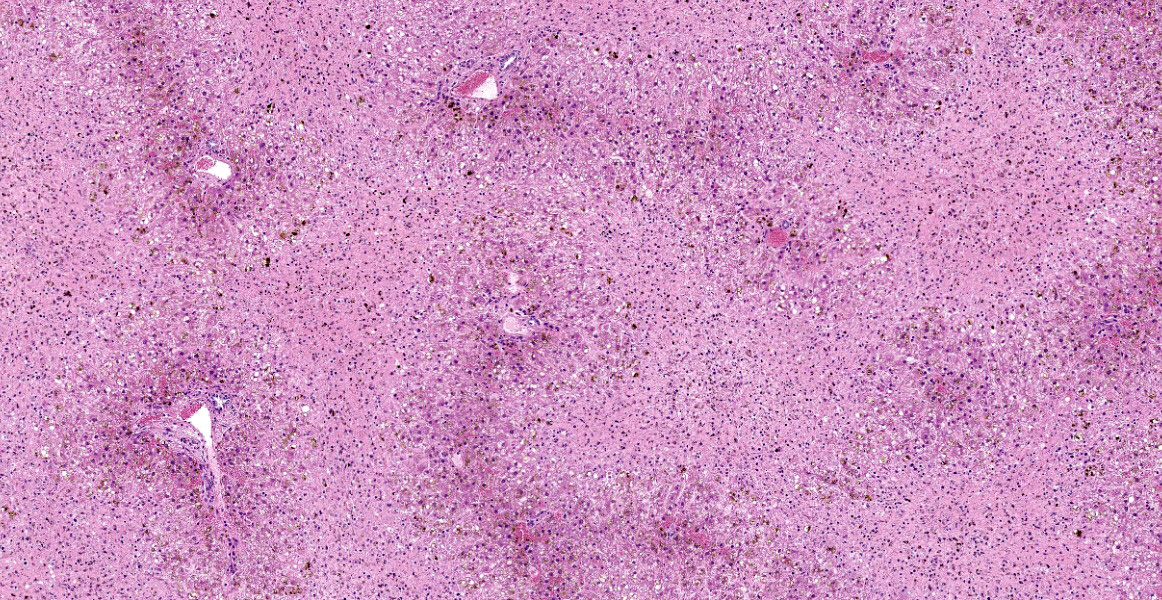
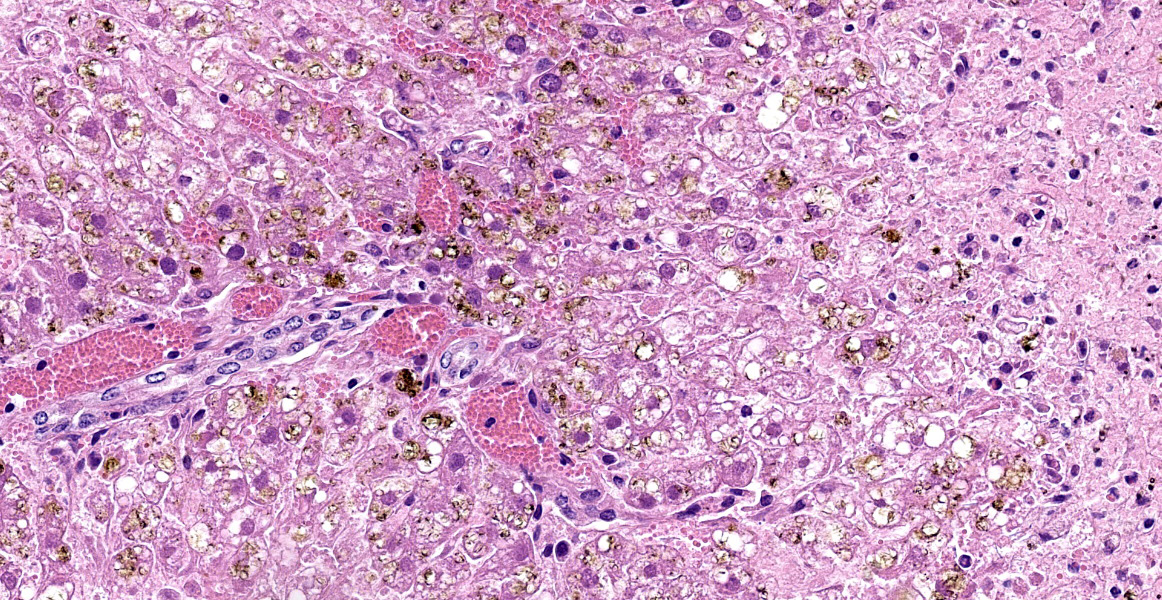
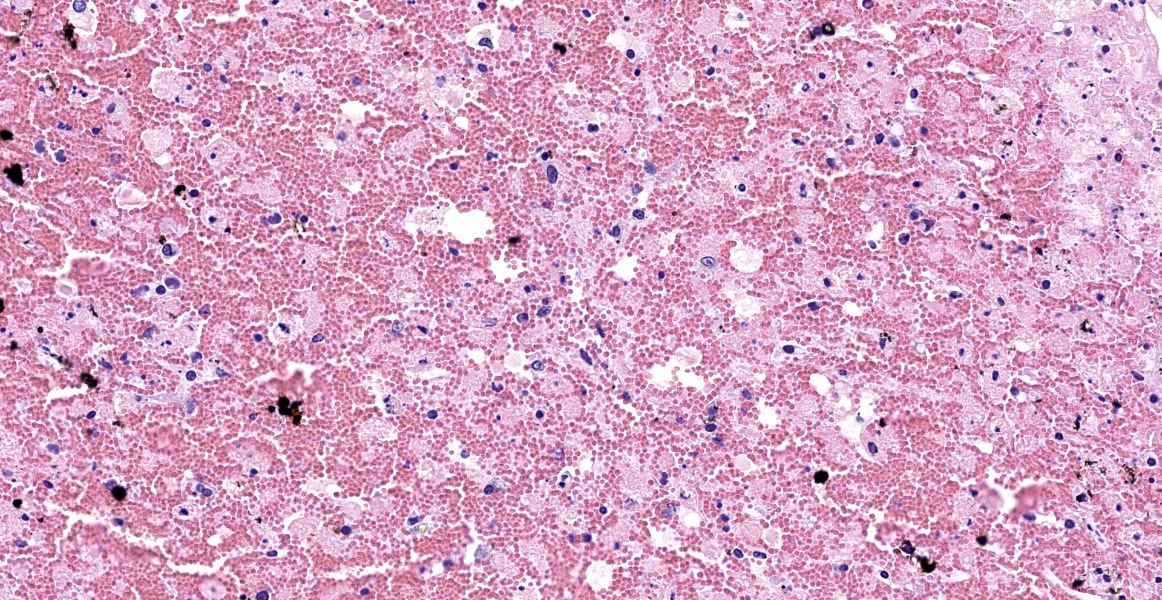
Liver: Diffusely, there is submassive to massive necrosis spanning periacinar, midzonal and frequently periportal regions. Necrosis is characterized by disruption and loss of normal hepatic sinusoidal architecture; shrunken, fragmented, hypereosinophilic hepatocytes with pyknotic, karyorrhectic or karyolytic nuclei; or complete loss of hepatocytes, replaced by extravasated erythrocytes (hemorrhage) and small amounts of cellular and karyorrhectic debris. Multifocal intact hepatocytes in periportal areas are swollen with vacuolated pale cytoplasm (degeneration), and rare limiting plate hepatocytes are normal.
Contributor’s Morphologic Diagnoses:
Liver: Severe acute submassive to massive hepatic necrosis
Contributor’s Comment:
Trema tomentosa, formerly known as Trema aspera, and colloquially known as poison peach, is an evergreen shrub to small tree of the family Ulmaceae, which is characterised by alternately attached leaves that are elliptical- to spearhead-shaped and dark green, with finely-toothed edges and hairy upper surfaces; small clusters of greenish-white flowers in the leaf-stem junctions; and round, fleshy, black, 3-4 mm berries.7 Poison peach is located on the east and north coasts of Australia, in the states of Victoria, New South Wales, Queensland, Northern Territory and the north coast of Western Australia.7
The leaves of poison peach contain a toxin known as trematoxin, which is an uncharacterised hepatotoxic glycoside, and is reported to cause fatal poisoning in cattle, sheep, goats, horses, and camels.4,6,9,10 Clinical signs of trematoxicosis can include recumbency, anorexia, muscle fasciculations, rigid posture, reluctance to move, dyspnoea, rapid weak pulse, and salivation.5,8,10 On post mortem examination, all reported cases have hepatic necrosis as the main feature, characterised as coagulative, periacinar to massive, and frequently with extensive hemorrhage.5,7,8,10 Further pathology described in the literature is predominately hemorrhage, including subcutaneous hemorrhages of the neck, chest and abdomen; petechial haemorrhages on the serosa of the rumen, abomasum and duodenum; and subepicardial and endocardial hemorrhages.6,8
Another species of the same genus, Trema micrantha, is located in the tropical to subtropical Americas, particularly South and Central America, and Florida in North America; and is reported to cause a similar disease in horses and goats.1,3 Additionally, there is evidence that ingestion of Trema micrantha by sheep can cause a fatal pneumotoxicosis, with the documented pathology including mediastinal emphysema, interalveolar septal thickening, and diffuse type II pneumocyte hyperplasia.11
As trematoxin is uncharacterized, definitive diagnosis of toxicity is difficult. Diagnosis is generally based on known exposure to the plant in combination with acute periacinar to massive hepatic necrosis. Differential diagnoses for acute hepatotoxicity in goats include amatoxins, cyanobacteria, Xanthium spp, green cestrum, cycads, gossypol, iron, polycyclic aromatic hydrocarbons, and carbon tetrachloride.4,7 Further, intoxication with Xanthium, green cestrum and Trema spp. cause very similar gross and microscopic changes, and therefore differentiating the three syndromes may involve isolation of the specific toxin where possible, or demonstration of ingestion of the plant species.3
In this case, no poison peach leaves or other toxic plant leaves were identified in the rumens of the two goats necropsied. It is hypothesized by the authors and submitting veterinarian that as the leaves are fine and palatable, they were well masticated and digested quickly, or were unidentifiable within the other ingesta of the rumen. There was no known exposure to other hepatotoxins aside from poison peach, and thorough examination of the pasture by the farm hand and submitting veterinarian did not reveal further hepatotoxic plants. Additionally, the sheep that were housed with the goats were not affected. Therefore, it was concluded that ingestion of poison peach was the most likely cause of hepatic necrosis and death in this herd of goats.
Contributing Institution:
The School of Veterinary Science
The University of Queensland, Gatton Campus
Queensland
Australia 4343
https://veterinary-services.lab.uq.edu.au/
JPC Diagnosis:
Liver: Necrosis, massive.
JPC Comment:
This case is a classic example of centrilobular hepatocellular necrosis. Centrilobular hepatocytes are particularly susceptible to toxic injury due to their high concentration of cytochrome P450 (CYP450) and susceptibility to hypoxia due to the location furthest downstream of the hepatic artery. 3 During phase I biotransformation and detoxification, CYP450 metabolism of toxins may cause formation of reactive intermediates with local toxic effects.3 Other examples of toxins which cause centrilobular necrosis are acetaminophen, microcystin, amanita, and Xanthium spp.3 Other less common patterns of hepatocellular necrosis are periportal hepatocellular necrosis, caused by direct acting toxins like yellow phosphorous, and midzonal necrosis, which is the least common.3
This week’s moderate, Dr. John Cullen, described the variability in histologic appearance between the different sections of liver on the slide. One possible explanation is a phenomenon called portal streaming, where blood from a specific portion of the gastrointestinal tract may be routed to specific areas of the liver through laminar flow, thus delivering different amounts of pathogens (whether toxic, infectious, or neoplastic). Other possible explanations include different the different volume of each load, some regulation of flow which redirects blood flow, or sampling technique. Dr. Cullen explained that similar variation between lobes is seen in copper deposition and this variation demonstrates why sampling all hepatic lobes is critical for accurate surgical biopsies.
The contributor described a related plant, Trema micrantha (Jamaican nettletree or capulin), which causes centrilobular necrosis in horses, goats, and sheep, and also causes pneumotoxicity in sheep. Ingestion of the plant, which appears to be highly palatable, can also cause acute neurotoxicosis without hepatotoxicosis in horses.2,6 A recent report described T. micrantha ingestion by 14 horses in Brazil with primary neurotoxicosis.6 Horses ingested the plant after being food restricted or having access to fresh trimmings and were ataxic with progressive neurologic signs resulting in death within one week. 6 On necropsy, the brains were dif fusely yellow with scattered foci of hemorrhage and necrosis, and the pons most severely affected. 6 Three horses had similar spinal cord lesions. On histology, there was multifocal fibrinoid necrosis of vessels, thrombosis, and liquefactive necrosis in the brainstem, cerebellum, and, less frequently, in the spinal cord. 6 Centrilobular hepatocellular necrosis was present in only five cases and was characterized as mild. 6 Horses with T. micrantha toxicity may also develop neurologic signs secondary to hepatotoxicity and hepatic encephalopathy.6 Histologically, these cases feature perivascular edema and Alzheimer type II astrocytes.2,6 These were not observed in the recent report, and the hepatic lesions were mild to absent, so hepatic encephalopathy was not suspected in these horses.6 The authors speculate that there may be intermediate metabolites which have species-specific toxic effects.6
References:
- Bandarra P, Bezerra P, De Oliveira L, et al. Experimental Trema micrantha (Cannabaceae) poisoning in horses. Pesqui Vet Brasil. 2011; 31(11): 991-996.
- Bandarra PM, Pavarini SP, Raymundo DL, Correa AMR, Pedroso PMO, Driemeier D. Trema micrantha toxicity in horses in Brazil. Equine Vet J. 2010; 42(5):456-459.
- Cullen JM, Stalker MJ. Liver and Biliary System. In: Maxie MG, ed. Jubb, Kennedy and Palmer's Pathology of Domestic Animals. Vol 2. 6th Ed. St. Louis, Missouri: Elsevier, 2016:258-352.
- Haschek WM, Rousseaux CG, Wallig MA. Fundamentals of toxicologic pathology. 2nd ed. Amsterdam, NL: Elsevier/Academic Press, 2010.
- Hill B, Wills L, Dowling R. Suspected poisoning of horses by Trema aspera (poison peach). Aust Vet J. 1985; 62(3): 107-108.
- Lorenzett MP, Pereira PR, Bassuino DM, et al. Neurotoxicosis in horses associated with consumption of Trema micrantha. Equine Vet J. 2018; 50: 192-195.
- McKenzie, RA. Australia's Poisonous Plants, Fungi and Cyanobacteria: a Guide to Species of Medical and Veterinary Importance. Collingwood, Vic: CSIRO Publishing; 2012. 635-637.
- Mulhearn C. POISON PEACH (TREMA ASPERA): A PLANT POISONOUS TO STOCK. Aust Vet J. 1942; 18(2): 68-72.
- Oelrichs P. Isolation and purification of trematoxin from Trema aspera. Phytochemistry. 1968; 7(9); 1691-1693.
- Trueman K, Powell M. Suspected poisoning of camels by Trema tomentosa (poison peach). Aust Vet J. 1991; 68(6): 213-214.
- Wouters F, Wouters ATB, Watanabe TTN, et al. Pneumotoxicosis in Sheep Caused by Ingestion of Trema Micrantha. Vet Pathol. 2013; 50(5), 775-778.