Signalment:
Gross Description:
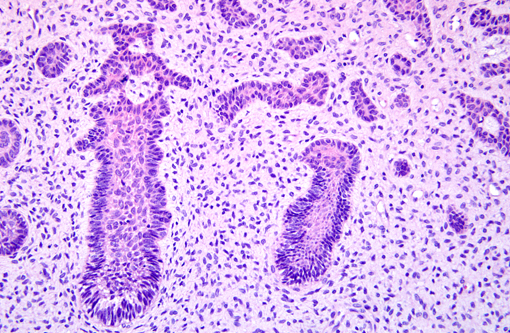
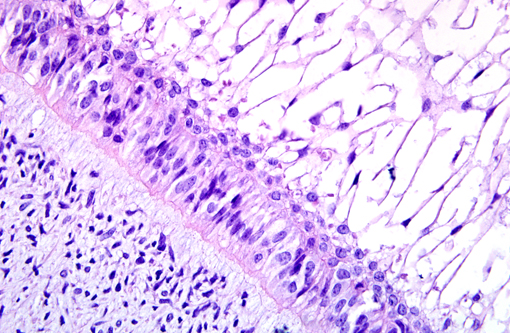
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Bacterial culture from gingival ulcer: Normal flora
Immunohistochemisty of pan-cytokeratin: odontogenic epithelial cells: +++
Immunohistochemisty of vimentin: mesenchymal cells: +++
Condition:
Contributor Comment:
In humans, ameloblastic fibroma (AF) belongs to a family of tumors with mixed proliferating odontogenic epithelium and a cellular mesenchymal component that resembles dental papilla. Members of this family include AF, ameloblastic fibroscarcoma (ameloblastic sarcoma), ameloblastic fibro-odontoma, odontoameloblastoma and odontoma, with or without dental hard tissue formation.(1,7-8) AF is generally intraosseous, mostly in young patients, more male than female.(3,6) The mandible is the predominant site of occurrence and the posterior mandible is affected more commonly than the maxilla.(6)
Grossly, AF occurs as solid, soft tissue mass with or without a capsule. Histologically, epithelial cells are forming anastomosing cords and/or islands. The cells at the periphery of the epithelial islands are columnar cells, with palisading nuclei and reverse nuclear polarity, similar to ameloblasts. The cells in the center of the epithelial islands had a stellate reticulum-like appearance, similar to the stellate reticulum of the enamel organ. The ectomesenchymal component comprised loose connective tissue, with abundant extracellular matrix containing young, plump fibroblasts, sometimes with a stellate appearance, with dendritic branches resembling the dental papilla. Microcyst formation is rarely seen. If there is dentin formation, the lesion should be diagnosed as ameloblastic fibrodentinoma; if there is also enamel formation, it should be diagnosed as ameloblastic fibro-odontoma.(1)
AF is a rare tumor in animals and has been reported in young horses and dogs, but it is the most common odontogenic tumor in cattle, in which, this neoplasm is similar to the lesions in humans and primarily composed of loose, collagen-poor primitive stroma resembling dental pulp. Odontogenic epithelial cells form cords and sheets, resembling enamel organ epithelium.(4)
AF must be distinguished from ameloblastomas, particularly those with desmoplastic changes. Distinct from AF, odontogenic epithelial cells in ameloblastoma are surrounded by mature fibrous connective tissue rather than primitive mesenchymal stroma.(4,7) Differentiation of ameloblastic fibrosarcoma from AF is also critical. Ameloblastic fibrosarcoma is the malignant counterpart of AF characterized by marked cytologic atypia, increased cellularity with diminution of the epithelial component, high numbers of mitoses and aggressive behavior.(10) Ameloblastic fibro-odontoma has features of AF, but, in addition, they have enamel and dentin deposition.(4) Anti-pan-cytokeratin antibody is suggested to detect odontogenic epithelial cells. Odontogenic epithelial cells are positive for cytokeratin, but negative to vimentin by immunohistochemistry. The mesenchymal component is positive for vimentin.(2)
JPC Diagnosis:
Conference Comment:
The enamel organ is composed of the outer enamel epithelium, inner enamel epithelium, stellate reticulum and stratum intermedium, which give rise to ameloblasts that produce enamel.Â
The dental papilla contains cells that develop into dentin-forming odontoblasts, and mesenchymal cells within are responsible for formation of tooth pulp.Â
Tooth development is commonly divided into the following stages: the bud stage, the cap, the bell, and maturation. The bud stage is characterized by the appearance of a tooth bud without a clear arrangement of cells. The tooth bud itself is the group of cells at the end of the dental lamina, and is formed when cells of the oral ectoderm proliferate faster than cells of other areas, and is best described as an in-growth of oral ectoderm. This dividing tissue is surrounded and stimulated by ectomesenchymal growth. When it is present, the dental lamina connects the developing tooth bud to the epithelium of the oral cavity. Eventually, the dental lamina disintegrates into small clusters of epithelium and is resorbed. This invagination of ectodermal tissues is the progenitor to the later ameloblasts and enamel while the ectomesenchyme is responsible for the dental papilla and later odontoblasts (8).Â
The first signs of an arrangement of cells in the tooth bud occur in the cap stage. A small group of ectomesenchymal cells stops producing extracellular substances, which results in an aggregation of these cells into the dental papilla. The tooth bud grows around the ectomesenchymal aggregation, taking on the appearance of a cap, and becomes the enamel organ. A condensation of ectomesenchymal cells called the dental follicle surrounds the enamel organ and limits the dental papilla, and later forms the surrounding alveolar bone and periodontal ligament (5).Â
The dental organ is bell-shaped during the bell stage, and the majority of its cells are stellate reticulum. Cells on the periphery of the enamel organ separate into three layers. Cuboidal cells on the periphery of the dental organ are the outer enamel epithelium, the columnar cells of the enamel organ adjacent to the dental papilla are the inner enamel epithelium, and the cells between the inner enamel epithelium and the stellate reticulum form the stratum intermedium. The rim of the dental organ where the outer and inner enamel epithelium join is called the cervical loop. In summary, the layers in order of innermost to outermost consist of dentin, enamel, which is formed by inner enamel epithelium, or ameloblasts, as they move outwards and upwards, inner enamel epithelium and stratum intermedium, which are the specialized stratified cells that support the synthetic activity of the inner enamel epithelium (5).Â
Hard tissues, including enamel and dentin, develop during the next stage of tooth development, which is the crown, or maturation stage. Mitosis stops during the crown stage at the location where the cusps of the teeth form, and the first mineralized hard tissues form at this location. At the same time, the inner enamel epithelial cells change in shape from cuboidal to columnar. The nuclei of these cells move closer to the stratum intermedium and away from the dental papilla. The adjacent layer of cells in the dental papilla increases in size and differentiates into odontoblasts, and form dentin. The odontoblasts secrete an organic matrix into their immediate surrounding which contains the material needed for dentin formation. As odontoblasts deposit organic matrix, they migrate toward the center of the dental papilla. Thus, unlike enamel, dentin starts forming in the surface closest to the outside of the tooth and proceeds inward. Cytoplasmic extensions are left behind as the odontoblasts move inward, which form the unique, tubular microscopic appearance of dentin (5).Â
After dentin formation begins, the cells of the inner enamel epithelium secrete an organic matrix against the dentin. This matrix immediately mineralizes and becomes the tooth enamel. Outside the dentin are ameloblasts, which continue the process of enamel formation; therefore, enamel formation moves outwards, adding new material to the outer surface of the developing tooth. Reciprocal induction governs the relationship between the formation of dentin and enamel; dentin formation must always occur before enamel formation. Generally, enamel formation occurs in two stages: the secretory and maturation stages. Proteins and an organic matrix form a partially mineralized enamel in the secretory stage, and the maturation stage completes enamel mineralization (5).Â
In the secretory stage, ameloblasts release enamel proteins that contribute to the enamel matrix, which is then partially mineralized by the enzyme alkaline phosphatase. Odontoblasts differentiate from cells of the dental papilla. They begin secreting an organic matrix around the area directly adjacent to the inner enamel epithelium, closest to the area of the future cusp of a tooth. The organic matrix contains collagen fibers with large diameters (0.10.2 μm). The odontoblasts begin to move toward the center of the tooth, forming an extension called the odontoblast process. Thus, dentin formation proceeds toward the inside of the tooth. The odontoblast process causes the secretion of hydroxyapatite crystals and mineralization of the matrix. This area of mineralization is known as mantle dentin (5).Â
There was some confusion among conference participants between the diagnosis of ameloblastic fibroma and ameloblastoma. Ameloblastic fibromas are well demarcated and consist primarily of a loose, matrix-poor primitive mesenchyme and sheets or cords of poorly formed odontogenic epithelium. Those ameloblastic fibromas with more advanced dentinal differentiation i.e. deposition of dentin or enamel matrix, are categorized as ameloblastic fibro- odontomas or odontoameloblastomas.Â
Ameloblastomas are usually discrete tumors with irregular islands and strands of odontogenic epithelium and an abrupt transition to mature fibrous stroma. They can occur in the gingival soft tissue (peripheral ameloblastoma) or deep in the bone of the jaw (central ameloblastoma). Additionally, keratinization can occur and be abundant within the odontogenic epithelium.Â
References:
2. Cardona A, Madewell BR, Naydan DK, Lund JK. A comparison of six monoclonal antibodies for detection of cytokeratins in normal and neoplastic canine tissues. J Vet Diagn Invest 1:316-323 (1989)
3. Fernandes AM, Duarte EC, Pimenta FJ, Souza LN, Santos VR, Mesquita RA, et al. Odontogenic tumors: a study of 340 cases in a Brazilian population. J Oral Pathol Med. 2005;34:583-7.Â
4. Head KW, Cullen JM, Duielzig RR, Else RW, Misdorp W, Patnaik AK, Tateyama S, van der Gaag I. Histological Classification of Tumors of the Alinetary System of Domestic Animals. 2nd ed., Washington, DC: Armed Forces Institute of Pathology; 1999:47-57
5. McGeady TA, Quinn PJ, Fitzpatrick ES, Ryan MT. Veterinary Embryology. Oxford, England:Wiley-Blackwell; 2006:279-282.Â
6. Olgac V, Koseoglu BG, Aksakalli N. Odontogenic tumours in Istanbul: 527 cases. Br J Oral Maxillofac Surg. 2006;44:386-8.Â
7. Press SG. Odontogenic tumors of the maxillary sinus. Curr Opin Otolaryngol Head Neck Surg 16:4754 8. Reichart PA, Philipsen HP. Ameloblastic fibroma. In: Odontogenic Tumors and Allied Lesions, London: Quintessence Publishing Co Ltd, 2004:121.Â
9. Sciubba JJ, Fantasia JE, Kahn LB. Development of the teeth and jaws. In: Rosai J, Sobin SH eds., Atlas of Tumor Pathology, Tumors and Cysts of the Jaw. 3rd ed. Washington, DC: Armed Forces Institute of Pathology; 1994: 1-5.Â
10. www.pathconsultddx.com. Ameloblastic fibrosarcoma. -