WSC 2023-2024, Conference 17, Case 3
Signalment:
3-year-old, male castrated mixed breed dog (Canis lupus familiaris)
History:
This dog initially presented to the submitting veterinarian for significant anxiety and urinating and defecating in the home.
Bloodwork was performed and revealed a marked eosinophilia (4,341 cells/µL, RR: 70-1,490 cells/ µL). Idexx SNAP test was negative for heartworm antigen, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. No additional diagnostics were pursued. At that time, the animal was started on Prozac for generalized anxiety; however, his condition continued to progress, and the owner began noticing additional sensory deficits (sight and smell) and incoordination. Approximately six months later, humane euthanasia was elected due to poor quality of life and increasing aggression toward the owner. The owner was bitten by the dog, resulting in rabies testing and a full necropsy.
Gross Pathology:
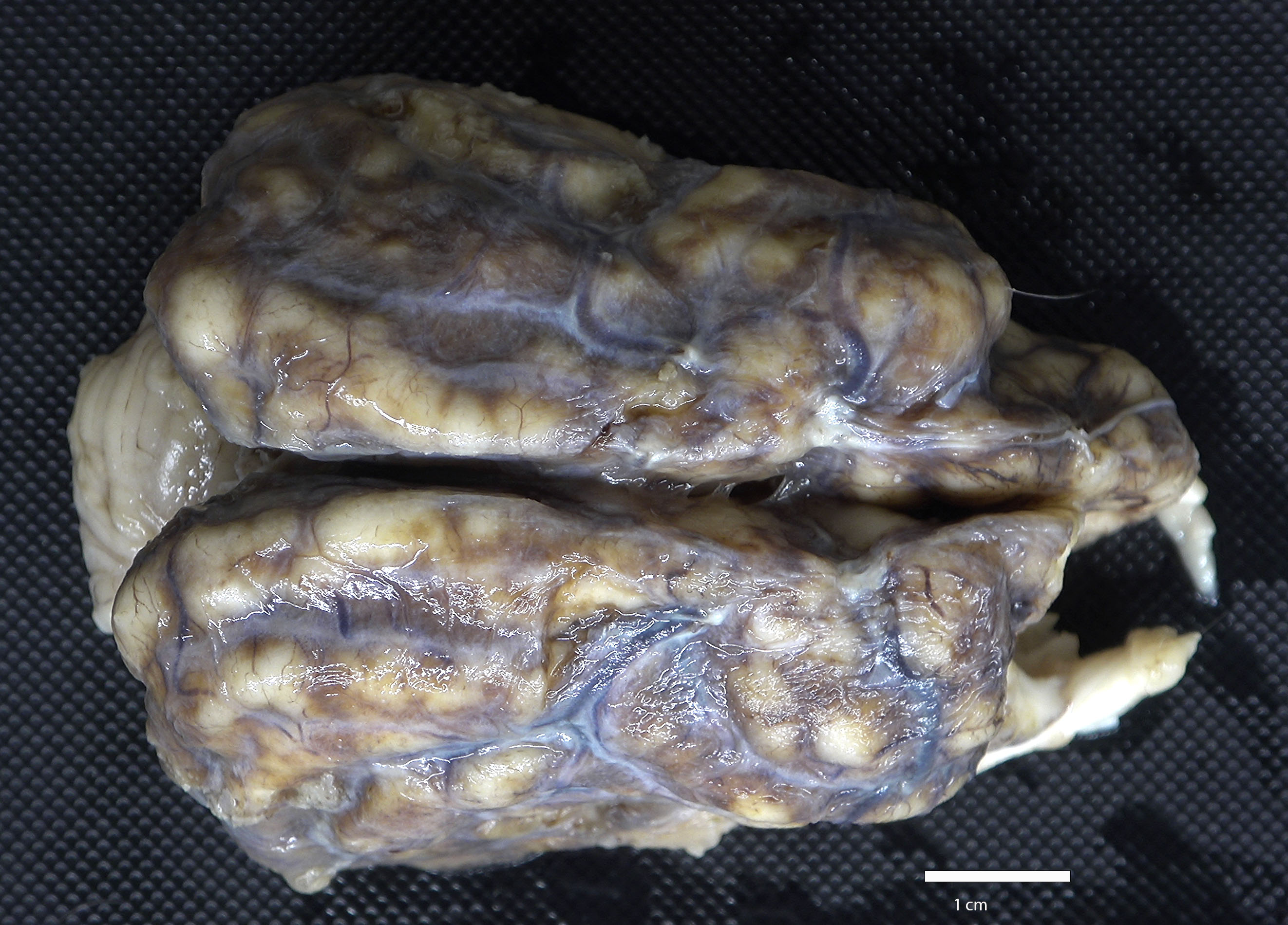
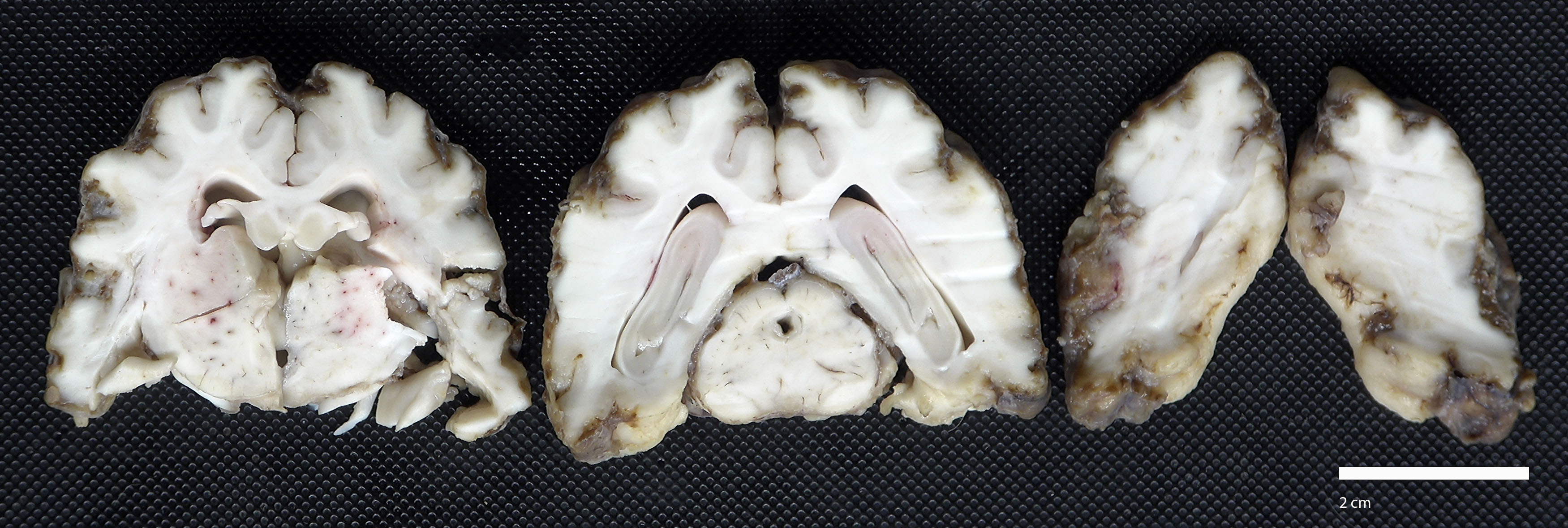
The cerebrum was semi-firm, lacked distinct sulci, and meninges were diffusely expanded by tan to dark brown exudate. On cut surface, the exudate extended into and replaced the outer layers of the cerebral cortex.
Laboratory Results:
Direct fluorescent antibody for rabies virus, brain: Negative
Neospora caninum PCR, brain: Not detected
Toxoplasma gondii PCR, brain: Not detected
Qualitative fecal analysis: Negative
Microscopic Description:
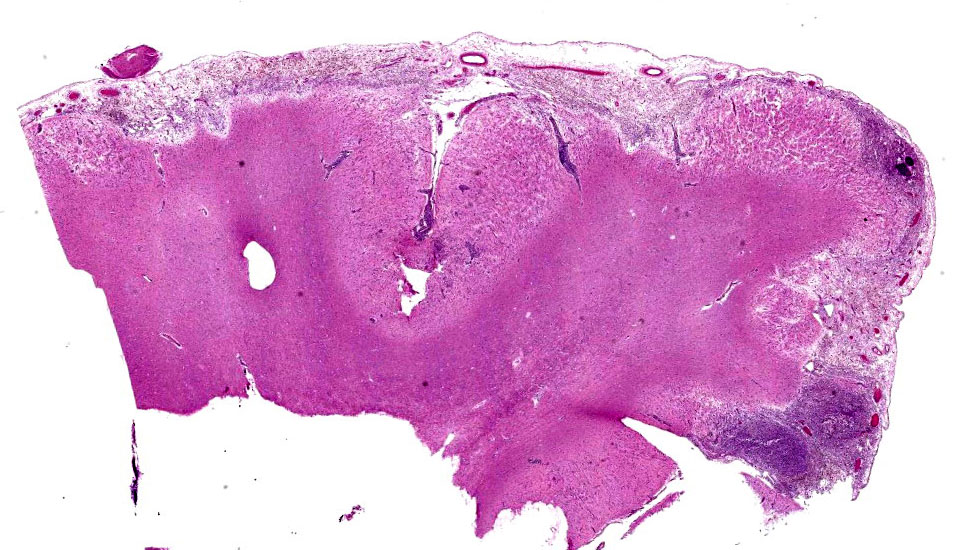
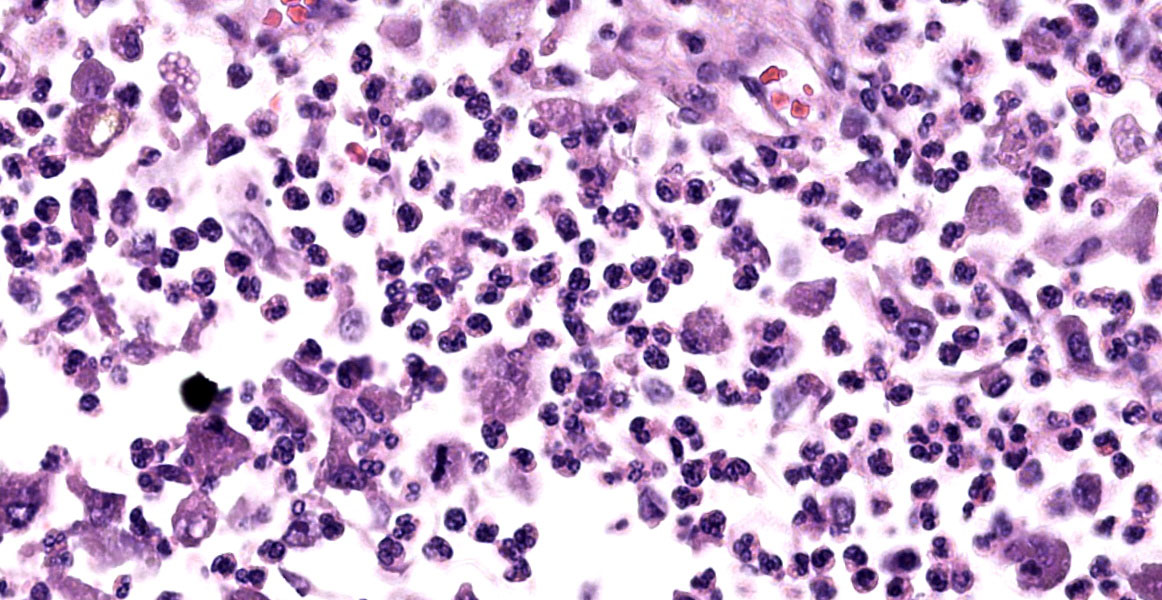
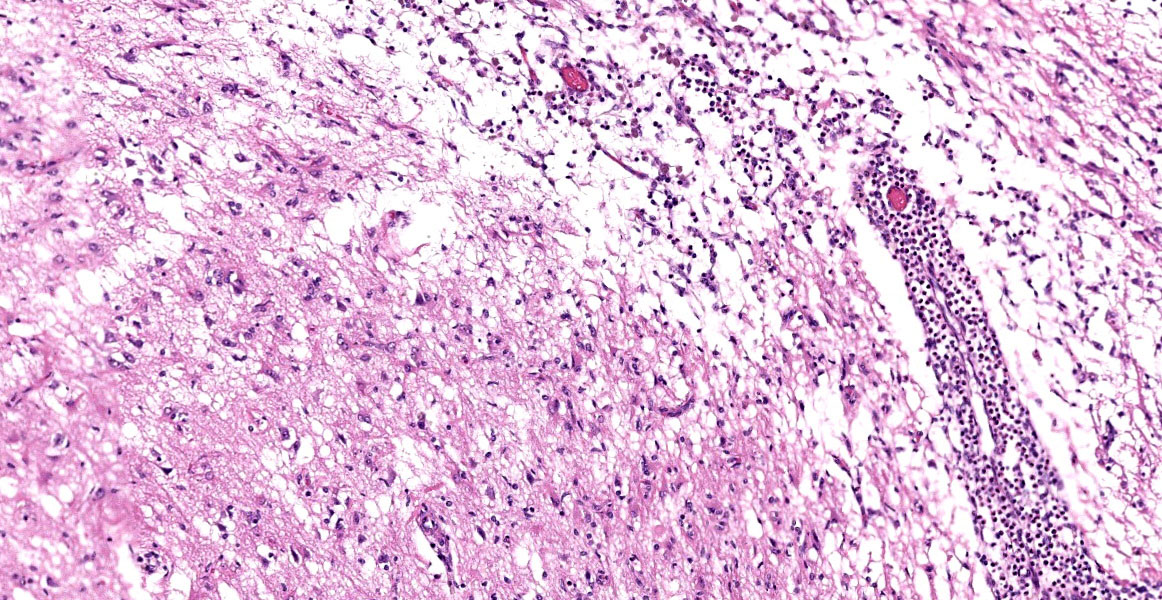
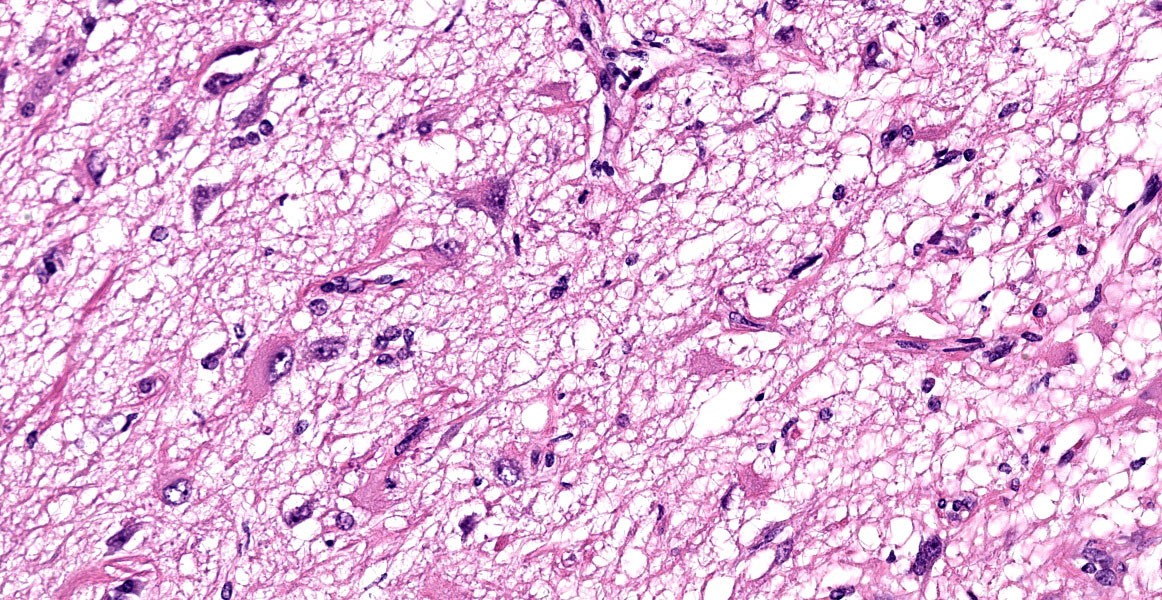
Brain: Meninges were markedly expanded by numerous eosinophils, hemosiderin-laden macrophages, fewer plasma cells, and rare multinucleated giant cells. This infiltrate extended into the cerebral gray matter and surrounded large regions of necrosis and rarefaction. The adjacent white matter contained low numbers of scattered spheroids and Virchow-Robin spaces were expanded by low numbers of lymphocytes and plasma cells. No infectious agents, infarction, or neoplastic cells were observed on hematoxylin and eosin, periodic acid-Schiff reaction, or Gram-stained sections.
Contributor’s Morphologic Diagnosis:
Brain: Severe, chronic, diffuse, eosinophilic and granulomatous to necrotizing meningoencephalitis with perivascular cuffing.
Contributor’s Comment:
This case is consistent with eosinophilic meningoencephalitis (EME), which is an uncommon to rare neurologic disease in humans and animals.2,3,8,12 In humans, EME is often associated with parasitic infections (such as Angiostrongylus cantonensis, Gnanthostoma spinigerum, cysticercosis (Taenia spp.), schistosomiasis, paragonimiasis, fascioliasis, and Toxocara canis) and occasionally tuberculosis, syphilis, coccidiodomycosis, and meningeal lymphoma.8,9,12
In veterinary medicine, EME is most often reported in dogs and has been associated with the following etiologies: Toxoplasma gondii, Neospora caninum, Cryptococcus spp., Prototheca spp., canine distemper virus, rabies virus, bacterial encephalitis, and aberrant migration of parasites (including Angiostrongylus spp. and Cuterebra spp).3,6,8,9,12,13 Other non-infectious causes of EME include infarction, trauma, or neoplasia.8,12 Additionally, if an underlying disease process cannot be identified, it is classified as idiopathic EME.3,12 Within the literature, idiopathic EME is predominantly noted in dogs, but has rarely been reported in cats, cattle, and sheep.2,3,6-13 Clinically, these dogs often present with mentation or behavioral changes, seizures, ataxia, blindness, and pain.3,6,8-10,12,13 On a routine diagnostic work-up, the only major abnormality may be peripheral eosinophilia, as seen in this case. Only about half of these cases have peripheral eosinophilia; therefore, cerebrospinal fluid analysis is considered more sensitive.3,12,13 On MRI, these cases have variable findings but often have bilateral, symmetrical lesions limited to the cerebral cortex that are associated with diffuse meningeal contrast uptake.3,13 In the reported case, neither CSF evaluation nor MRI was performed.
On postmortem examination, the meninges are often reported to appear thickened and green.12 However, this was not observed in our case, likely due to the abundant amount of hemosiderin within the macrophages. Microscopically, the leptomeninges of the brain and spinal cord in cases of EME are frequently infiltrated by eosinophils, macrophages, and other mononuclear cells, and there is a variable amount of parenchymal loss, degeneration, and necrosis within the underlying cortical grey matter with multifocal regions of perivascular cuffing.8-10,12
The CNS, particularly neurons and myelinated axons, are highly susceptible to eosinophilic-induced neurotoxicity.8,12 Eosinophils are often activated in association with chronic inflammatory conditions (asthma, chronic allergen, etc.) by helper T (Th2) cells releasing IL-4, IL-5, IL-3, and granulocyte-macrophage colony-stimulating factor (GM-CSF).5,6,8,12 Once activated, eosinophils release major basic protein, eosinophil cationic protein, eosinophil peroxidase, and oxygen free radicals which aid in killing parasites.2,5,6,10,12 However, they are also neurotoxic and can cause severe tissue damage within the CNS.12 In the reported case, the absence of overt infectious agents, parasites, infarction, trauma, or neoplasia, combined with the ancillary testing, make a diagnosis of idiopathic EME most likely.
The exact pathogenesis of idiopathic EME is unknown. Idiopathic EME is reported more often in young to middle-aged, large breed dogs.2,8,10,12,13 Additionally, rottweilers and golden retrievers appear to be over-represented in the population of reported cases, suggesting a possible breed predisposition.2,6,9,10,12,13
Many of the reported cases of idiopathic EME had minor to drastic improvement in the patient’s condition when treated with corticosteroids, which has led to the hypothesis that there is an underlying immune-mediated or hypersensitivity process.2,3,6-9,12 It is important to note that this improvement could be due to the normal pathophysiological interactions of steroids in the body rather than treating a specific underlying disease process, as steroids can induce eosinophil apoptosis and reduce the number within the blood.5 Mayhew IG, et al. discussed a case of EME in a dog with suspected underlying hypersensitivity or drug-induced (firocoxib) reaction.7 Additional cases with extensive clinical and pathological evaluation are necessary to further understand the underlying etiology and prognosis of idiopathic EME.
Contributing Institution:
Michigan State University
Veterinary Diagnostic Laboratory
4125 Beaumont Rd, Lansing, MI 48910
https://cvm.msu.edu/vdl
JPC Diagnosis:
Cerebrum: Meningoencephalitis, eosinophilic, superficial, marked, with cortical necrosis.
JPC Comment:
Dogs play host to a wide range of diseases, including EME, for which the contributor provides an excellent summary, that are characterize histologically by the accumulation of eosinophils. Canine eosinophilic pulmonary granulomatosis and eosinophilic bronchopneumopathy were discussed earlier this conference year, and insights on these conditions can be found in WSC #9, case 2. Other canine eosinophilic diseases include eosinophilic granuloma; eosinophilic furunculosis; eosinophilic esophagitis, gastritis, and gastroenteritis; eosinophilic cystitis; and hypereosinophilic syndrome.
Canine eosinophilic granuloma is an uncommon skin disease that frequently presents as papules, nodules, or plaques, most frequently in the oral cavity.4 Canine eosinophilic granuloma is attributed to hypersensitivity reactions to insect bites or environmental and food allergens, though some bacterial and fungal agents have been implicated.4 There is a pronounced breed predilection in Siberian husky dogs and Cavalier King Charles Spaniels, suggesting a genetic basis in at least some cases.4
Eosinophilic gastrointestinal disease (EGID) is an umbrella terms encompassing a spectrum of disorders characterized by eosinophilic inflammation in one or more sites such as the esophagus, stomach, intestine, and colon.1 Diagnosis of EGID requires abnormal numbers and distributions of eosinophils in the gastrointestinal tract in the absence of other underlying etiologies such as parasitism.1 Some studies have noted that the number of identifiable eosinophils in tissue section is likely an undercount, as degranulated eosinophils are easily overlooked or confused with other granulocytes. Immunohistochemical staining for eosinophil peroxidase, which highlights both viable and degranulated eosinophils, have revealed a four to 40-fold increase in eosinophil number over H&E evaluation alone, depending on the examined tissue.1 As in the rest of the eosinophilic entities, EGID is thought to be a Th-2 driven hypersensitivity reaction likely, in the case of EGID, due to food allergens.1
The moderator began discussion with a broad overview of possible causes for eosinophilic encephalitis and meningoencephalitis, including parasitic involvement, protozoal infection, hypersensitivity reactions, and the breed specific presentations noted by the contributor. Dr. Miller noted the striking subgross histologic appearance of this slide, with the eosinophil-induced neuroparenchymal necrosis giving the cerebral profile an unusual, scalloped appearance. Dr. Miller noted that the tissue loss focally extends into the white matter in the examined section and, as such, the term neuropil, which refers exclusively to gray matter, would be an inappropriate descriptor here; neuroparenchyma, encompassing both gray and white matter, would be the correct term to refer to the extent of the tissue loss.
The neuroparenchymal loss and eosinophilic inflammation present in section are both more extensive than typically seen with EME and more closely resemble lesions associated with parasitism. Dr. Miller noted that Cuterebra larval migration, relatively common in cats and occasionally occurring in dogs, has a very similar histologic presentation. Neuroparenchymal loss caused by Cuterebra is due to an exotoxin secreted by the larvae that leads to necrosis of adjacent neuroparenchyma. The lesion is both necrotizing and eosinophil-rich, often with concurrent vasculitis, and larvae are frequently not identified in histologic section. Dr. Miller provided examples of the gross and histologic appearance of feline ischemic encaphlopathy caused by Cutererba larval migration and reminded participants to keep this differential in mind when presented with this histologic picture.
Consideration of the morphologic diagnosis for this case sparked robust discussion. Participants noted the numerous histiocytes within the examined section and considered whether to include “granulomatous” or “histiocytic” to characterize the inflammation. Participants decided to omit the histiocytic component as the pathogenesis here is likely eosinophil-driven, with histiocytes called in secondarily to repair the damage. Participants also struggled to capture the extent and distribution of the striking lesions. After much discussion, participants felt that “superficial” best conveyed the location of the inflammation within the meninges and in the outermost portion of the cortex. A few participants doggedly argued for the term “cortical loss” to capture the unique appearance of the cerebral contour; however, as the loss was caused by necrosis, participants felt that “cortical necrosis,” while perhaps not as punchy, was most appropriate.
References:
- Bastan I, Rendahl AK, Seelig D, et al. Assessment of eosinophils in gastrointestinal inflammatory disease in dogs. J Vet Intern Med.
- Bennett PF, Allan FJ, Guilford WG, et al. Idiopathic eosinophilic meningoencephalitis in Rottweiler dogs: three cases (1992-1997). Aust Vet J. 1997;75:786-789.
- Cardy TJA, Cornelis I. Clinical presentation and magnetic resonance imagining findings in 11 dogs with eosinophilic meningoencephalitis of unknown aetiology. J Small Anim Pract. 2018;59(7):422-431.
- Knight EV, Shipstone MA. Canine eosinophilic granuloma of the digits treated with prednisolone and chlorambucil. Vet Dermatol. 2016;27(5):446-e119.
- Kumar V, Abbas AK, Aster JC. Robbins & Cotran Pathologic Basis of Disease. 10th ed. Elsevier;2020.
- Lillihook I, Gunnarsson L, Zakrisson G, Tvedten H. Disease associated with pronounced eosinophilia: a study of 105 dogs in Sweden. J Small Anim Pract. 2000; 41:248-253.
- Mayhew IG, Hill KE, Ahn Y, Jones BR. Can drug-induced aseptic meningitis account for some cases of eosinophilic meningitis/meningoencephalitis in dogs? N Z Vet J. 2022;70(3):184-185.
- Olivier AK, Parkes JD, Flaherty HA, Kline KL, Haynes JS. Idiopathic eosinophilic meningoencephalomyelitis in a Rottweiler dog. J Vet Diagn Invest. 2010; 22(4):646-648.
- Smith-Maxie LL, Parent JP, Rand J, et al. Cerebrospinal fluid analysis and clinical outcome of eight dogs with eosinophilic meningoencephalomyelitis. J Vet Intern Med. 1989;3:167-174.
- Sykes JE, Weiss DJ, Buoen LC, et al. Idiopathic hypereosinophilic syndrome in 3 Rottweilers. J Vet Intern Med. 2001;15: 162-166.
- Vidana B, Floyd T, Brena C, et al. First case of idiopathic eosinophilic meningoencephalitis in a sheep. J Comp Pathol. 2020;174:58-62
- Williams JH, Koster LS, Naidoo V, et al. Review of idiopathic eosinophilic meningitis in dogs and cats, with a detailed description of two recent cases in dogs. J S Afr Vet Assoc. 2008;79(4):194-204.
- Windsor RC, Sturges BK, Veranu KM, Vernau W. Cerebrospinal fluid eosinophilia in dogs. J Vet Intern Med. 2009;23: 275-281.