Signalment:
Gross Description:
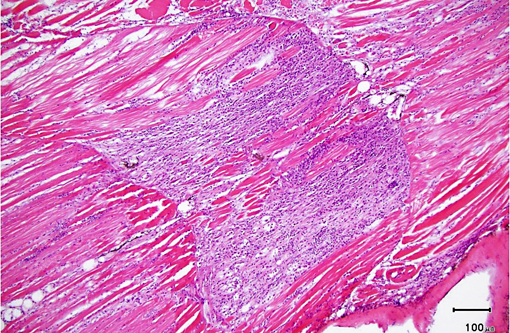
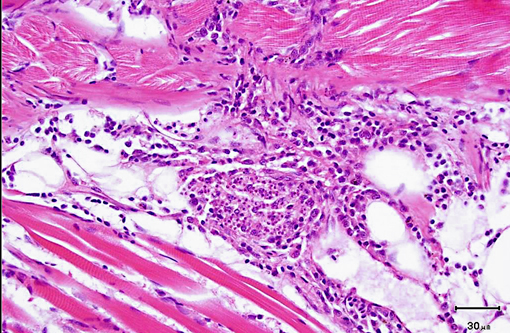
Histopathologic Description:
Morphologic Diagnosis:
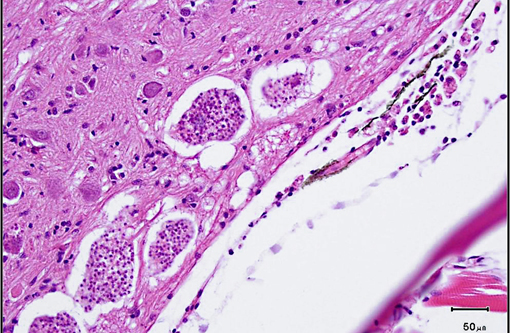
1. Brain, spinal cord: Microsporidian xenomas, few, morphology consistent with Pseudoloma neurophilia.
2. Nerve roots: Radiculoneuritis, histiocytic, multifocal, mild, with intralesional microsporidan spores (morphology consistent with Pseudoloma neurophilia).
Condition:
Contributor Comment:
Xenomas, which may measure up to 200 μm in diameter, are composed of several aggregates of up to 16 uninucleate spores segregated within sporophorous vesicles. Spores are oval to pyriform and measure approximately 5.5 x 2.5 μm. Like other microsporidians, the spores contain a polar filament. In addition to mature spores, sporophorous vesicles contain a few developmental stages known as sporoblasts. Unlike many other microsporidia, developmental stages are in direct contact with the host cytoplasm, rather than within parasitophorous vacuoles, based on electron microscopic studies.(5)
As was the case in this fish, a few xenomas usually do not stimulate a significant inflammatory response; however, large numbers of xenomas and/or the presence of free spores within tissues may be associated with significant inflammation. In heavily infected fish, the inflammatory reaction may extend from the spinal cord meninges or nerve roots into the adjacent skeletal musculature, where it may be associated with massive chronic inflammation and myodegeneration, with relatively few organisms present (either within xenomas or free spores which undergo phagocytosis by macrophages).Â
Transmission is primarily through the ingestion of infective spores, either free in the water or within the tissues of cannibalized fish. Experimental transmission following exposure of fish to water contaminated with organisms derived from infected spinal cords resulted in infection as early as 8 weeks post-exposure, with 100% of exposed fish infected by week 20.(4) As its name implies, P. neurophilia has a tendency to infect the central nervous system (particularly the brainstem and spinal cord), as well as nerve roots. However, both xenomas and free spores have been identified in other tissues, particularly the ovary and developing follicles, leading to speculation that vertical transmission (or at least transmission via sexual products released during spawning) may occur. Because of this and the fact that routine chlorine bleaching does not appear to kill the infective spores,(1) techniques involving re-derivation of colonies by the screening of adult fish using histopathology and/or of a percentage of their offspring by PCR have been developed and are being used by some facilities (including ours) in order to create SPF lines.(3) In addition to being used to test adults and eggs, PCR may be used to test water filtrates, biofilms, and other samples.(8) Histopathologic diagnosis is greatly facilitated by the use of a Luna stain, which binds to chitin and stains spores bright orange.(6) Other histochemical stains, such as Gram, Fites acid-fast, and Giemsa are variably effective in staining spores. Spores may also be visualized using fluorescent stains (Fungi-Fluor).(4) No treatment is currently available,(3) and infections persist following exposure, with no evidence for immune clearance by the host.(7)
JPC Diagnosis:
1. Brain: Microsporidial xenomas, multiple.
2. Spinal cord: Ganglioneuritis, histiocytic, multifocal, mild, with intracytoplasmic microsporidian spores.
Conference Comment:
References:
1. Ferguson JA, Watral V, Schwindt AR, Kent ML. Spores of two fish microsporidia (Pseudoloma neurophilia and Glugea anomala) are highly resistant to chlorine. Dis Aquat Org. 2007;76:205-214.Â
2. Keeling PJ, McFadden GI. Origins of microsporidia. Trends Microbiol. 1998;6(1):1923.
3. Kent ML, Buchner C, Watral VG, Sanders JL, LaDu J, Peterson TS, Tanguay RL. Development and maintenance of a specific pathogen-free (SPF) zebrafish research facility for Pseudoloma neurophilia. Dis Aquat Org. 2011;95:73-79.
4. Kent ML, Bishop-Stewart JK. Transmission and tissue distribution of Pseudoloma neurophilia (Microsporidia) of zebrafish, Danio rerio (Hamilton). J Fish Dis. 2003;26:423-426.
5. Matthews JL, Brown AMV, Larson K, Bishop-Stewart JK, Rogers P, Kent ML. Pseudoloma neurophilia n. g., n. sp., a new microsporidian from the central nervous system of the zebrafish (Danio rerio). J Eukaryot Microbiol. 2001;48:227-233.
6. Peterson TS, Spitsbergen JM, Feist SW, Kent ML. Luna stain, an improved selective stain for detection of microsporidian spores in histologic sections. Dis Aquat Org. 2011;95:175-180.
7. Ramsay JM, Watral V, Schreck CB, Kent ML. Pseudoloma neurophilia infections in zebrafish Danio Rerio: effects of stress on survival, growth, and reproduction. Dis Aquat Org. 2009;88:69-84.Â
8. Whipps CM, Kent ML. Polymerase chain reaction detection of Pseudoloma neurophilia, a common microsporidian of zebrafish (Danio rerio) reared in research laboratories. J Am Assoc Lab Ani Sci. 2006;45:13-16.Â