Signalment:
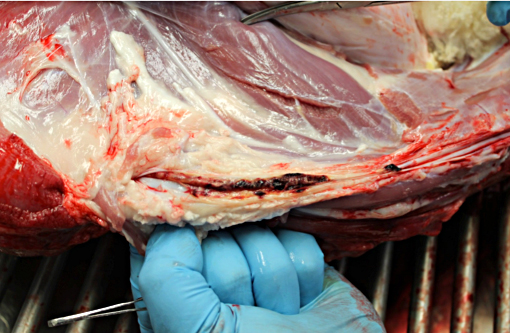
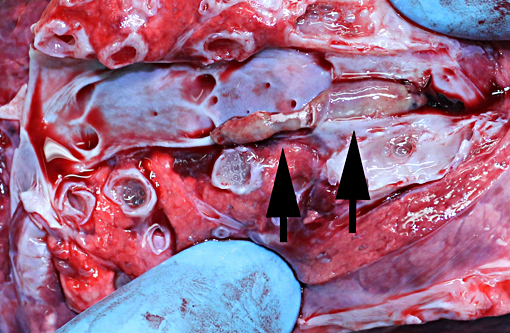
Gross Description:
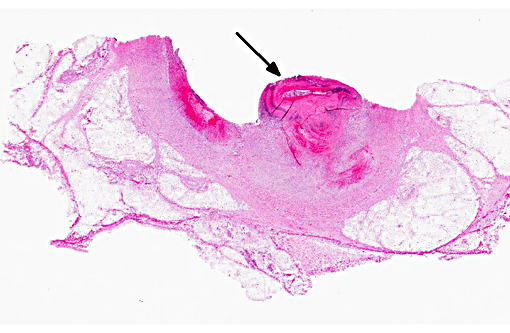
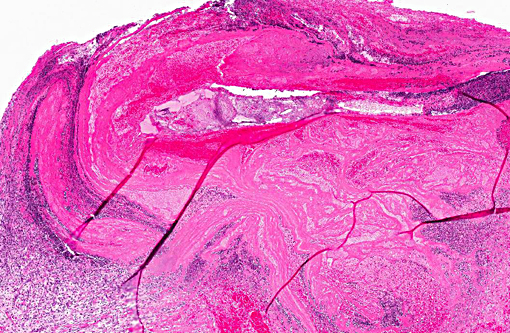
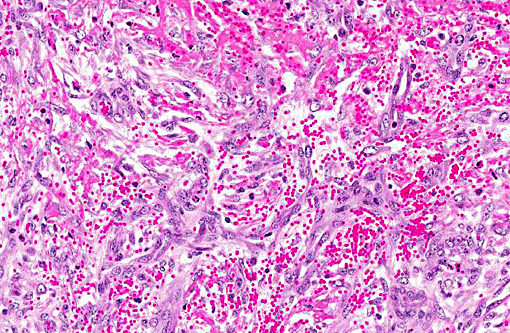
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Jugular thrombophlebitis can be a significant problem in ruminants, both related to catheterization as well as perivascular administration of irritating solutions (such as 5% dextrose for ketosis or calcium gluconate for milk fever). Broken off fragments from the jugular site were able to travel to the right heart before lodging in a pulmonary artery (thromboembolism). The distinction between bland and septic thromboemboli can be of critical importance, as the latter may give rise to additional foci of infection, including embolic pneumonia or nephritis.
The inherent resistance of Pseudomonas aeruginosa to chlorhexidine-based disin-fectants is well-documented.(3,4) Pseudomonas aeruginosa is a normal inhabitant of water systems, and is therefore nearly ubiquitous in distribution. The organism is a significant cause of hospital acquired infections, often with a poor prognosis related both to the resistance of the organism to treatment as well as comorbidities in the patients. Pseudomonas aeruginosa colonization of medical devices is facilitated by pili and fimbriae as well as biofilm formation, the latter making an-tibiotic treatment unrewarding.(1) Single blood cultures are frequently negative in cases of bacteremia, and frequent repeated large volume blood cultures have the best success.
JPC Diagnosis:
Conference Comment:
Hemostasis was reviewed in detail during the conference including a discussion of initiating events. While turbulent blood flow, a component of Virchows triad, is clearly present with the placement of an intraluminal catheter, endothelial injury is also a very important factor in the formation of a thrombus. Endothelial injury results in the exposure of tissue factor, and other subendothelial components such as collagen, resulting in platelet aggregation and the initiation of coagulation. The release of tissue factor, located within the plasma membrane of activated endothelium, results in the initiation of the extrinsic coagulation pathway; the intrinsic pathway is initiated by collagen and other subendothelial com-ponents coming into contact with pre-kallikrein, high molecular weight kininogen and factors XII and XI (contact group of coagulation factors).(2) When tissue factor comes into contact with factor VII, it forms a complex which along with calcium, activates factor X to initiate the common pathway. While conceptually it is easier to learn and discuss the coagulation cascade as two separate pathways which combine to form the common pathway, the in vivo process is more commonly considered a single intertwined set of events that begins with the exposure of tissue factor. (2)
Participants reviewed the basic steps in the hemostatic process starting with vasoconstriction and formation of a platelet plug, followed by coagulation and formation of a fibrin meshwork, followed by fibrinolysis and finally tissue repair, and the role of platelets in this process was discussed. Platelets adhere to exposed subendothelial collagen and von Willebrands factor is released by local activated endothelium, which provides a more secure connection between the collagen and platelets via platelet receptor GPIb. Platelets then release the contents of their α-granules and produce other mediators which continue to promote hemostasis. The release of adenosine diphosphate (ADP) results in the binding of fibrinogen to platelet receptor GPIIb-IIIa, and the fibrinogen forms a scaffold as the platelets aggregate, eventually covering the defect. Factors released from the aggregated platelets, such as platelet derived growth factor, stimulate fibroblast recruitment which can eventually result in fibrosis at the thrombus location, as occurred in this case. (2)
References:
1. Laverty G, Gorman SP, Gilmore BF: Biomolecular Mechanisms of Pseudomonas aeruginosa and Escherichia coli Biofilm Formation. Pathogens 2014:3(3):596-632.
2. Mosier DA: Vascular Disorders and Thrombosis. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. Fourth ed. St. Louis, Mo: Mosby Elsevier; 2007: 61-87.
3. Nakahara H, Kozukue H: Isolation of chlorhexidine-resistant Pseudomonas aeruginosa from clinical lesions. J Clin Microbiol 1982:15(1):166-168.
4. Oie S, Kamiya A: Microbial contam-ination of antiseptics and disinfectants. Am J Infect Control 1996:24(5):389-395.
5. van Vleet JF, Ferrans VJ: Cardiovascular System. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. Fourth ed. St. Louis, MO: Mosby Elsevier; 2007: 559-611.