CASE 3: 17-0359 (JPC 4136401-00)
Signalment:
10-year-old, female spayed, domestic longhair cat, Felis catus, feline
History:
Presented for a 5-day history of anorexia and lethargy, with two episodes of vomiting 2 days prior to presentation. The cat was not drinking for several days and was reported to be previously healthy.
Gross Pathology:
The body was received in good postmortem
and fair nutritional condition, with mild diffuse muscle atrophy. The skin,
subcutaneous tissues, mucous membranes, and sclera were severely diffusely
discolored yellow.
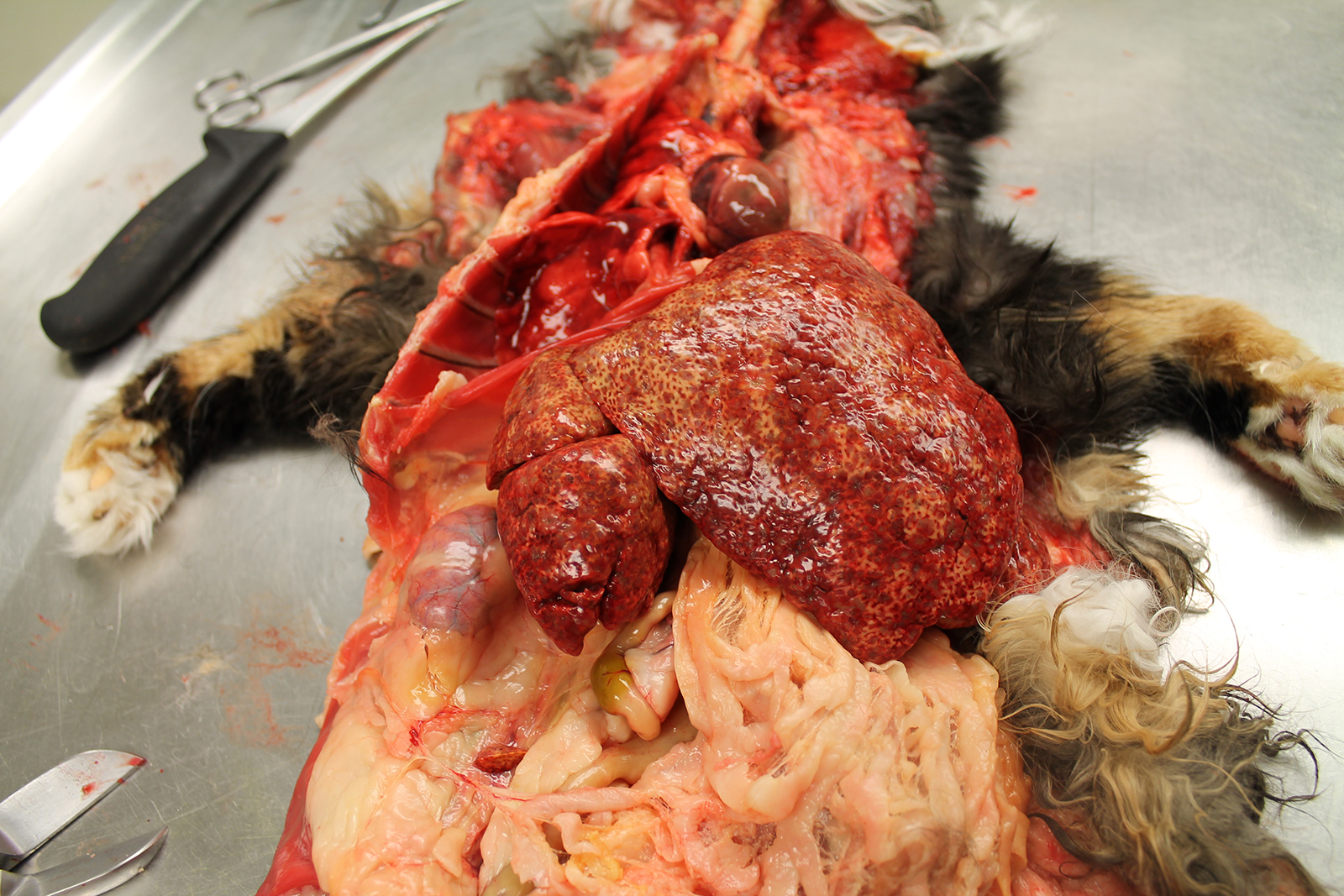
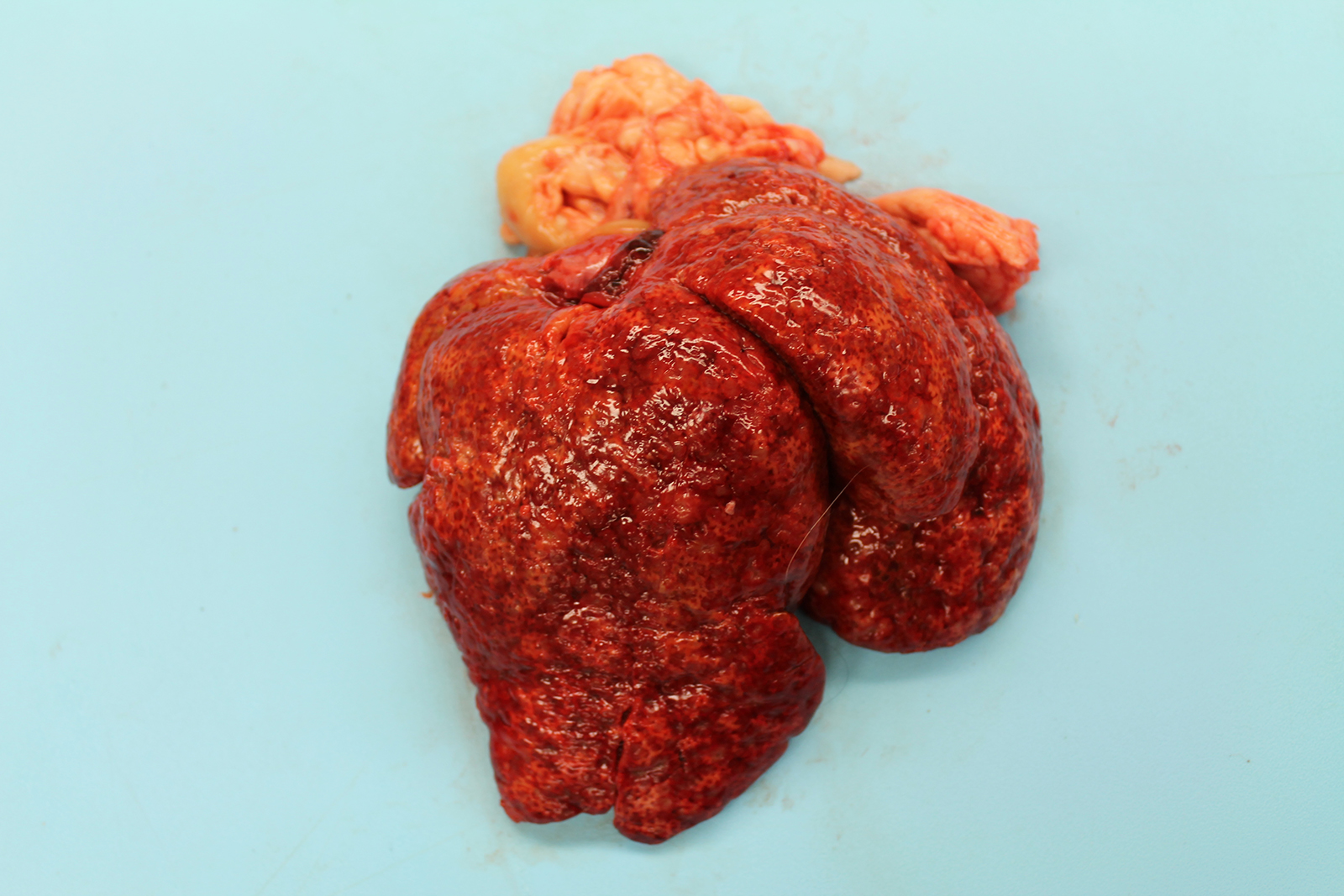
The liver was markedly enlarged (475g; 13.5% of body weight), firm, and mottled red-brown to white-tan with a slightly enhanced reticular pattern. There were too numerous to count irregular pinpoint to 0.2 cm grey depressions that occasionally contained trace amounts of serosanguineous fluid. On cut section, there were multifocal targetoid tan lesions with brown centers ranging from 0.2 to 0.6 cm diameter, almost completely effacing normal parenchyma.
The remainder of the gross examination was unremarkable.
Laboratory results:
None provided.
Microscopic description:
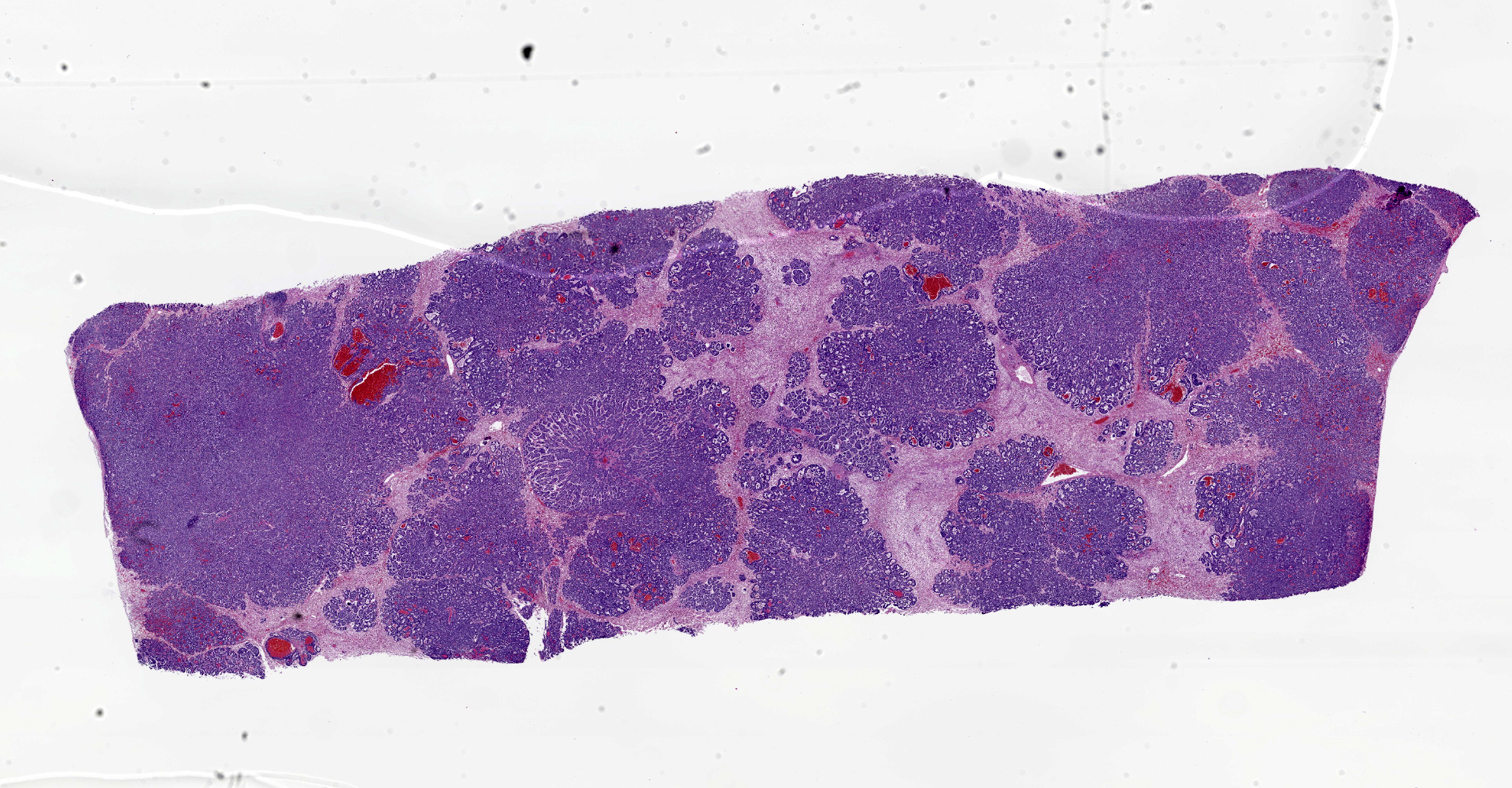
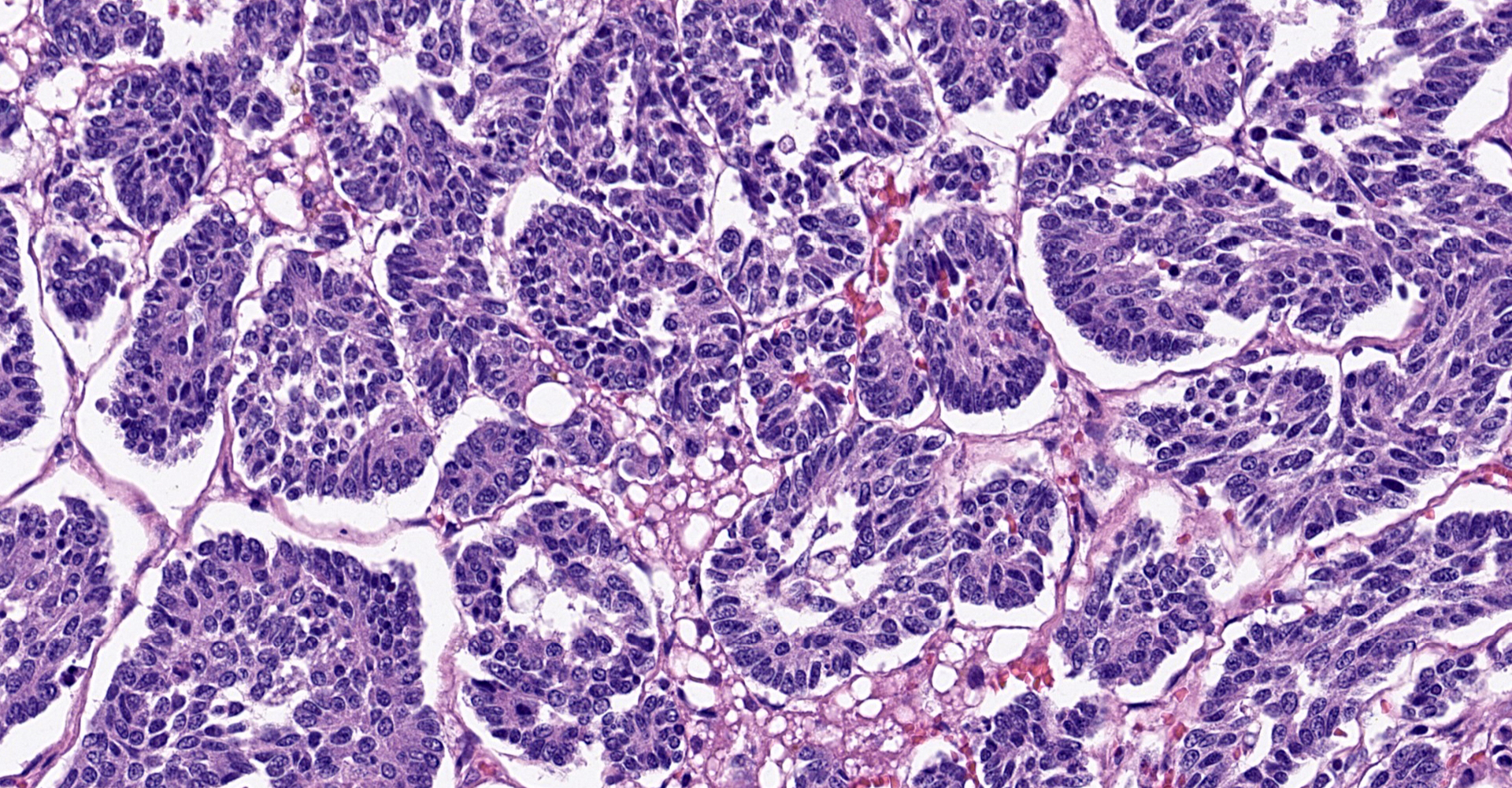
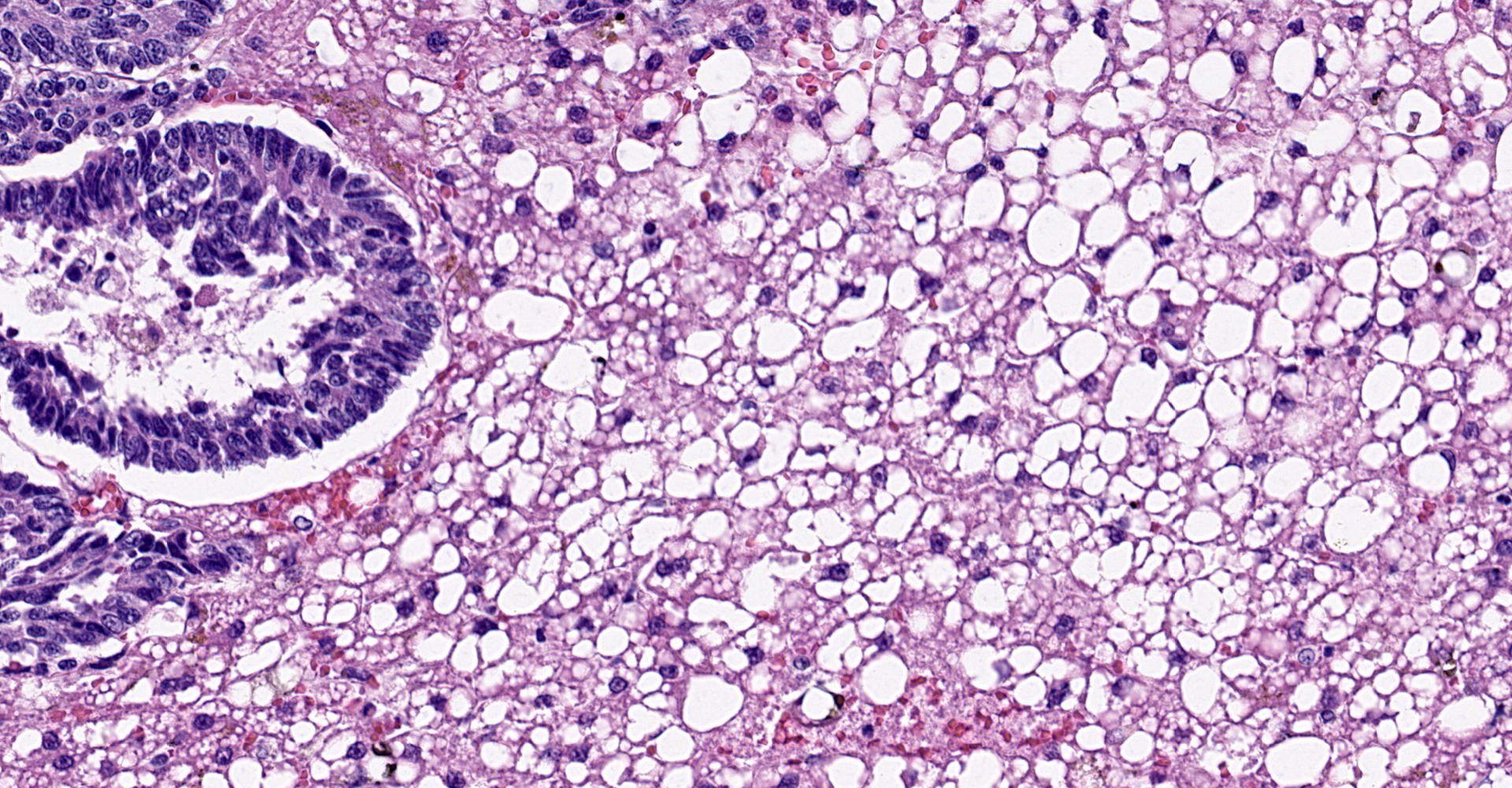
Liver: The normal liver architecture is approximately 80-90% replaced and effaced by an unencapsulated and infiltrative neoplasm composed of polygonal cells arranged in nests, packets, and cords, supported by a fine fibrovascular stroma. The neoplastic cells display occasional attempts at tubule formation and rosettes. Cell borders are distinct, and there is a moderate amount of eosinophilic to finely granular cytoplasm, round to oval often basilar nuclei with finely stippled chromatin and generally one prominent nucleolus. Mitoses are rare at less than 1 per single 400x field. There are small, scattered areas of necrosis and hemorrhage. Remaining small islands of hepatocytes are markedly compressed by the neoplasm and contain single large cytoplasmic lipid vacuoles which often peripheralize the nucleus (lipidosis).
No sites of metastasis were identified.
Contributor's morphologic diagnosis:
Hepatic neuroendocrine carcinoma with severe hepatic lipidosis, domestic longhair, feline.
Contributor's comment:
Neuroendocrine carcinomas (formerly "carcinoids") are malignant neoplasms that arise from the dispersed neuroendocrine system cells. These cells arise from the endoderm during embryogenesis. Neuroendocrine cells belong to a group of cells which secrete both peptide and non-peptide hormones (previously referred to as the amine precursor uptake and decarboxylation (APUD)) group. These cells are important in regulation of gastrointestinal function, and their products include catecholamines and gastrin.
Gastrin-producing tumors lead to gastric hypersecretion and gastric or duodenal ulceration, known as Zollinger-Ellison syndrome.11,14 Ulcers have been documented in some cats with neuroendocrine carcinoma with hepatic or extrahepatic neuroendocrine carcinoma15, but were not observed in this case. A recent report describes gastrin immunohistochemical positivity in a feline hepatic neuroendocrine carcinoma.7 Gastrin secretion by neuroendocrine tumors is well-documented in domestic species.3,11,14,15 An analogous syndrome in humans, carcinoid syndrome, is characterized by flushing, sweating, diarrhea, vomiting, abdominal pain, and heart failure, but this syndrome has not been documented in domestic animals to our knowledge.8
Common primary locations for these tumors
in cats and dogs include the gastrointestinal tract, gallbladder, and liver. While
they are overall rare in domestic animals, primary hepatic neuroendocrine
carcinomas in cats are documented in only a few reported cases.4,5,7,15
The present case represents the most common, though non-specific, clinical
signs on presentation of cats with hepatobiliary neuroendocrine carcinoma
(anorexia, weight loss, vomiting, and hepatomegaly). Most cases reported are
mature to senior on presentation, and breeds reported have been variable. Neuroendocrine
tumors have also been reported in an African pygmy hedgehog, cow, and others9,12
and a primary hepatic neuroendocrine carcinoma was recently reported in a
baboon.1
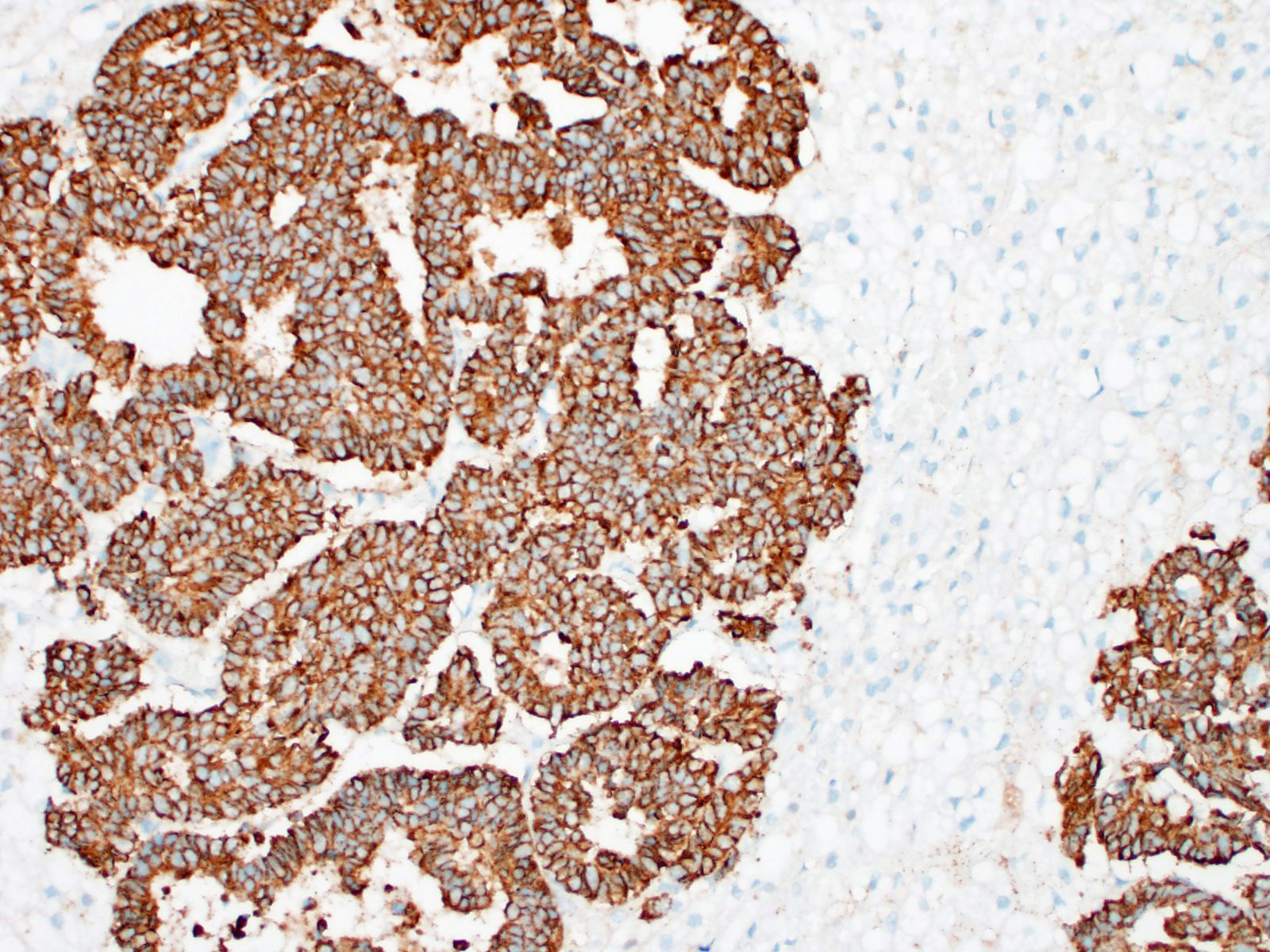
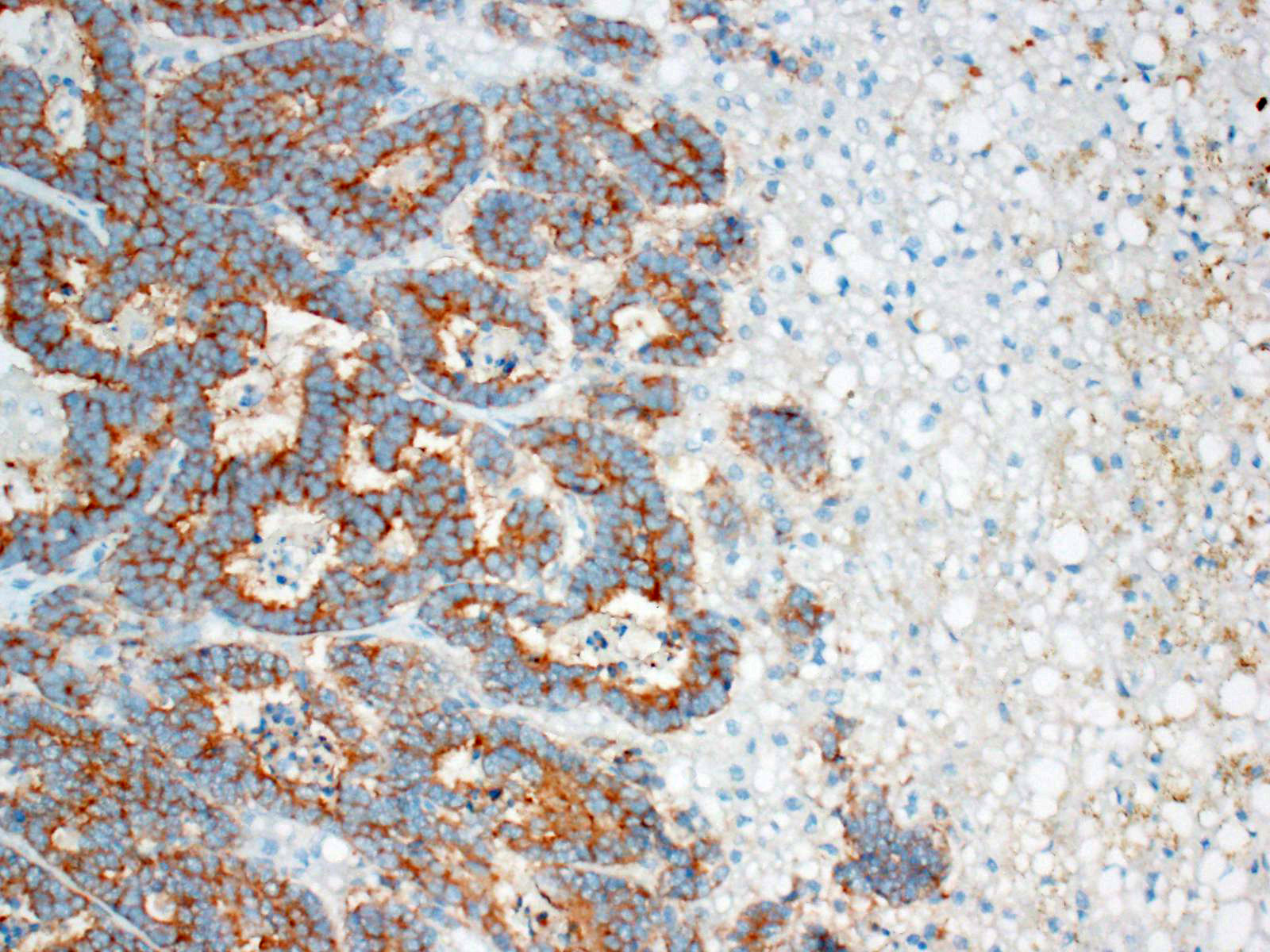
These tumors typically stain positively with neuron-specific enolase (NSE) and
synaptophysin, with variably positivity for chromogranin A.7,15 A
recent report describes gastrin positivity in a feline hepatic neuroendocrine
carcinoma7, further highlighting the potential for Zollinger-Ellison
syndrome occurring secondary to tumors outside the gastrointestinal tract.3,11,14,15
Other useful markers include Churukian-Schenk and Grimelius silver stains to
highlight argyrophilic cytoplasmic granules.11 Immunohistochemistry
was not performed in this case; however, the histomorphologic features were
considered sufficient for the diagnosis. An important differential diagnosis in
this case was cholangiocellular carcinoma, but the frequently observed rosettes
and palisading helped to rule out this differential.
Contributing Institution:
University
of Pennsylvania
https://www.vet.upenn.edu/
JPC diagnosis:
Liver: Neuroendocrine carcinoma (carcinoid acceptable).
JPC comment:
The moderator led a discussion about nomenclature regarding this entity. In the past, "carcinoid" was used to mean a neoplasm of neuroendocrine origin. However, there is value in being able to convey to a clinician the most likely biologic behavior of the neoplasm. "Carcinoma" is a familiar term for clinicians and conveys a great deal of meaning regarding a diagnosis of malignancy. In this case, the histologic features support a diagnosis of neuroendocrine "carcinoma" and we are confident in sharing that diagnosis with the surgeon or internist.
Neuroendocrine carcinoma is diagnosed infrequently in many species, primarily in the intestine, liver, lung, esophagus, skin, and nasal cavity. Neuroendocrine carcinomas have recently been described in sika deer16, flamingos2, a Japanese macaque6, and bearded dragons.9
Neoplasia is rarely reported in bearded dragons (Pogona vitticeps), but a recent case of gastric neuroendocrine carcinoma presented with similar clinical signs as mammalian neuroendocrine carcinomas. This neoplasm was poorly differentiated and was not immunoreactive to chromogranin A, nor gastrin; however, it was immunoreactive for somatostatin, and weakly immunoreactive for NSE.9
Unfortunately, there is no standard of care for treatment of these neoplasms. These are often invasive carcinomas, with diffuse infiltration, with limited ability for complete excision. A case of canine hepatic neuroendocrine carcinoma was treated with doxorubicin and metronomic cyclophosphamide treatment, which was tolerated well. The patient lived approximately 15.5 months from initial presentation. However, because these neoplasms are rare, and treatment is not standardized, it is not possible to determine whether the chemotherapy regimen increased this patient's survival time.13
References:
1. Aloisio F, Dick EJ Jr, Hubbard GB: Primary hepatic neuroendocrine carcinoma in a baboon (Papio sp.). J Med Primatol 38:23-26, 2009
2. Buckles EL. Phoenicopteriformes. In: Terio KA, McAloose D, St. Leger J, eds. Pathology of Wildlife and Zoo Animals. San Diego:Elsevier. 2018:692.
3. Cullen JM, Stalker MJ. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Vol 2. 6th ed. St. Louis, MO: Elsevier; 2016:349.
4. Ferreira-Neves, Patricia, et al. "Immunohistochemical Characterization of a Hepatic Neuroendocrine Carcinoma in a Cat." Journal of Veterinary Diagnostic Investigation, vol. 20, no. 1, 2008, pp. 110?114., doi:10.1177/104063870802000125.
5. Head KW, Cullen JM, Dubielzig RR, Else RW, Misdorp W, Patnaik AK, Tateyama S, van der Gaag I. Tumors of the Alimentary System of Domestic Animals, 2nd series.Washington D.C.: Armed Forces Institute of Pathology; 2003:33, 79, 94-96,127-128.
6. Hirata A, Miyamoto Y, Kaneko A, et al. Hepatic neuroendocrine carcinoma in a Japanese macaque (Macaca fuscata). Journal of Medical Primatology. 2018;48(2):137-140.
7. Kita, Chiaki, et al. "A Feline Case of Hepatic Neuroendocrine Carcinoma with Gastrin Immunoreactivity." Journal of Veterinary Medical Science, vol. 76, no. 6, 2014, pp. 887?890., doi:10.1292/jvms.13-0581.
8. Lips, C. J., et al. "The Spectrum of Carcinoid Tumours and Carcinoid Syndromes." Annals of Clinical Biochemistry, vol. 40, no. 6, 2003, pp. 612?627., doi:10.1258/000456303770367207.
9. Lowden LR, Davies JL. Malignant Neuroendocrine Tumor (Carcinoid) of the Spleen in an African Pygmy Hedgehog (Atelerix albiventris). J Comp. Path. 2016:155:88-91.
10. Lyons JA, Newman SJ, Greenacre CB, Dunlap J. Gastric neuroendocrine carcinoma expressing somatostatin in a bearded dragon (Pogona vitticeps). J Vet Diag Invest. 2010;22:316-320.
11. Michishita M, Takagi M, Kishimoto TE, et al. Pancreatic neuroendocrine carcinoma with exocrine differentiation in a young cat. J Vet Diagn Invest. 2017:29(3):325-330.
12. Michishita M, Takahashi K, Moriya H, Nakamura S, Koyama H, Sako T. Poorly differentiated rectal carcinoid in a cow. Vet Pathol. 2007;44:414-417.
13. Morgan E, O'Connell K, Thomson M, Boyd S, Sandy J. Primary hepatic neuroendocrine carcinoma treated with doxorubicin and cyclophosphamide in a dog. J Am Anim Hosp Assoc. 2019;55(3):e55305.
14. Munday JS, Lohr CV, Kiupel M. Tumors of the Alimentary Tract. In: Meuten J, ed. Tumors in Domestic Animals. 5th ed. Ames, Iowa: John Wiley & Soncs, Inc; 2017:559,568.
15. Patnaik AK, Lieberman PH, Erlandson RA, Antonescu C: Hepatobiliary neuroendocrine carcinoma in cats: a clinicopathologic, immunohistochemical, and ultrastructural study of 17 cases. Vet Pathol 42:331-337, 2005
16. Shibata R, Machida Y, Hatakeyama H, et al. Hepatic neuroendocrine carcinoma with metastases to the lymph nodes in a sika deer (Cervus nippon yakushimae). The Journal of Veterinary Medical Science. 2020; 82(2):193-196.
17. Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Jubb,
Kennedy and Palmer's Pathology of Domestic Animals. Vol 2. 6th ed. St. Louis, MO: Elsevier; 2016:105-106