Signalment:
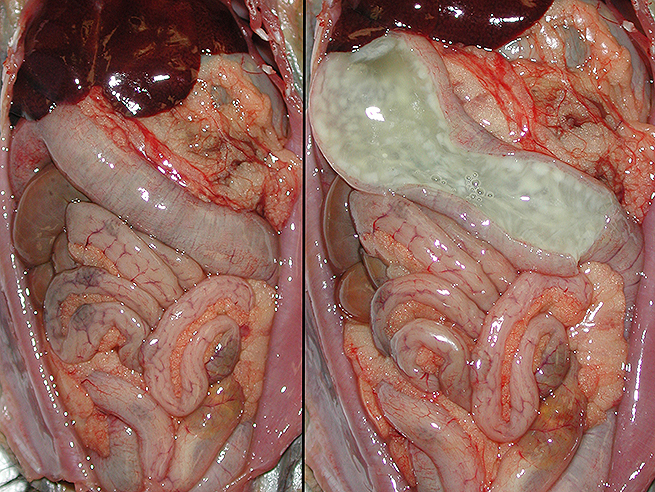
Gross Description:
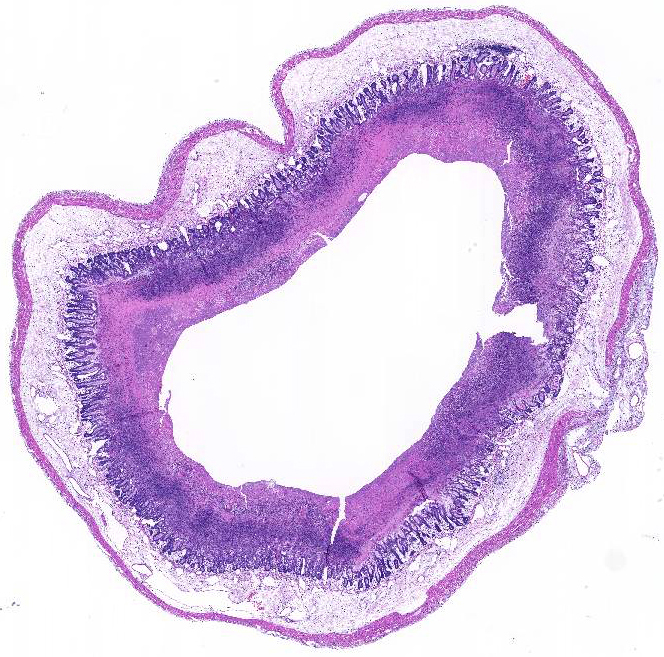
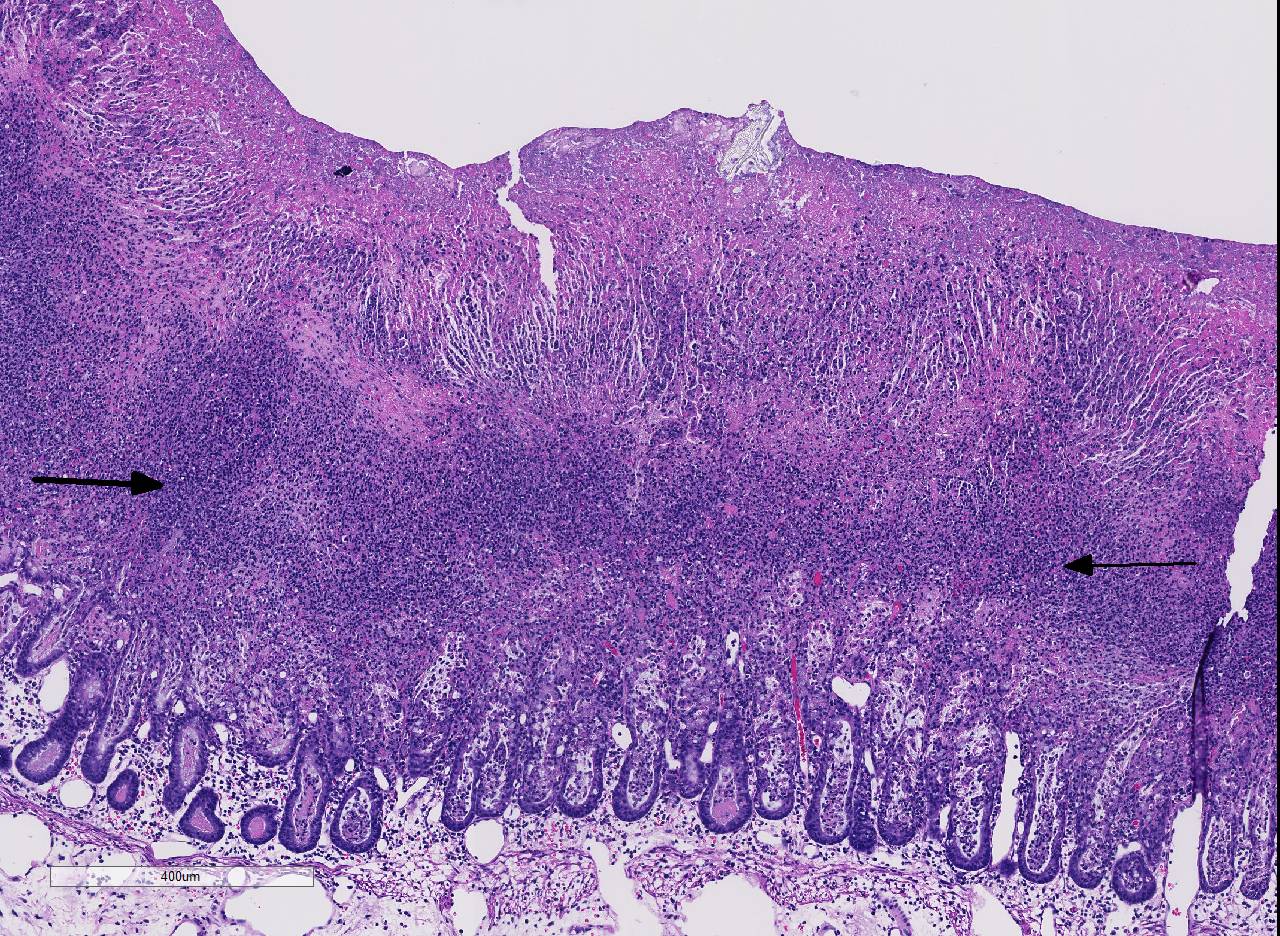
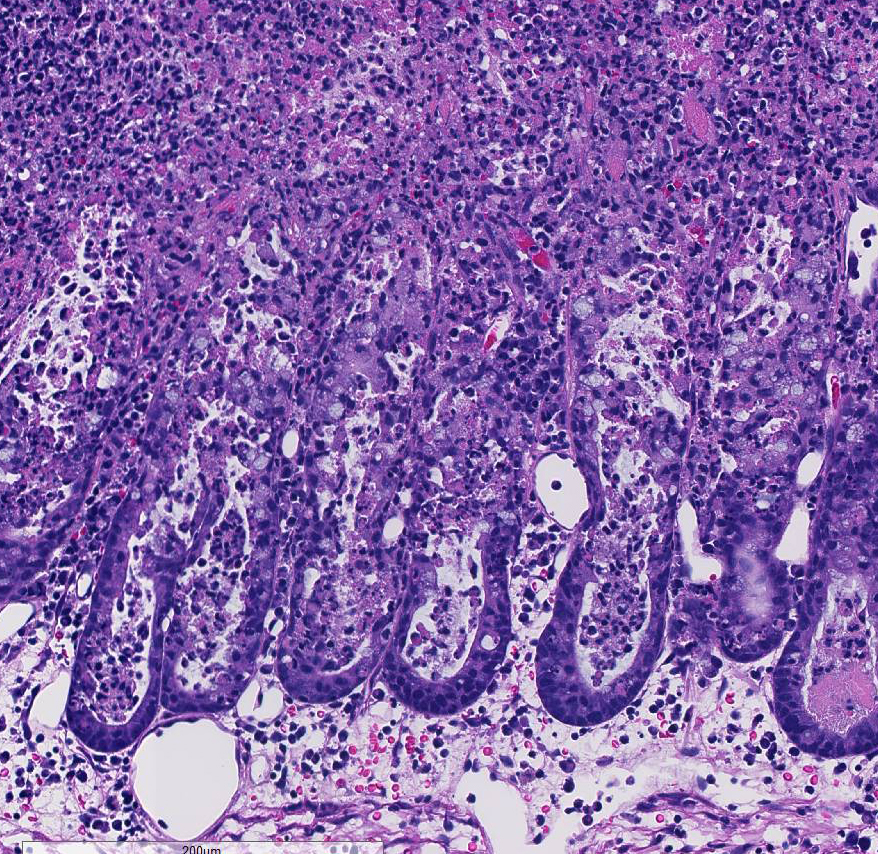
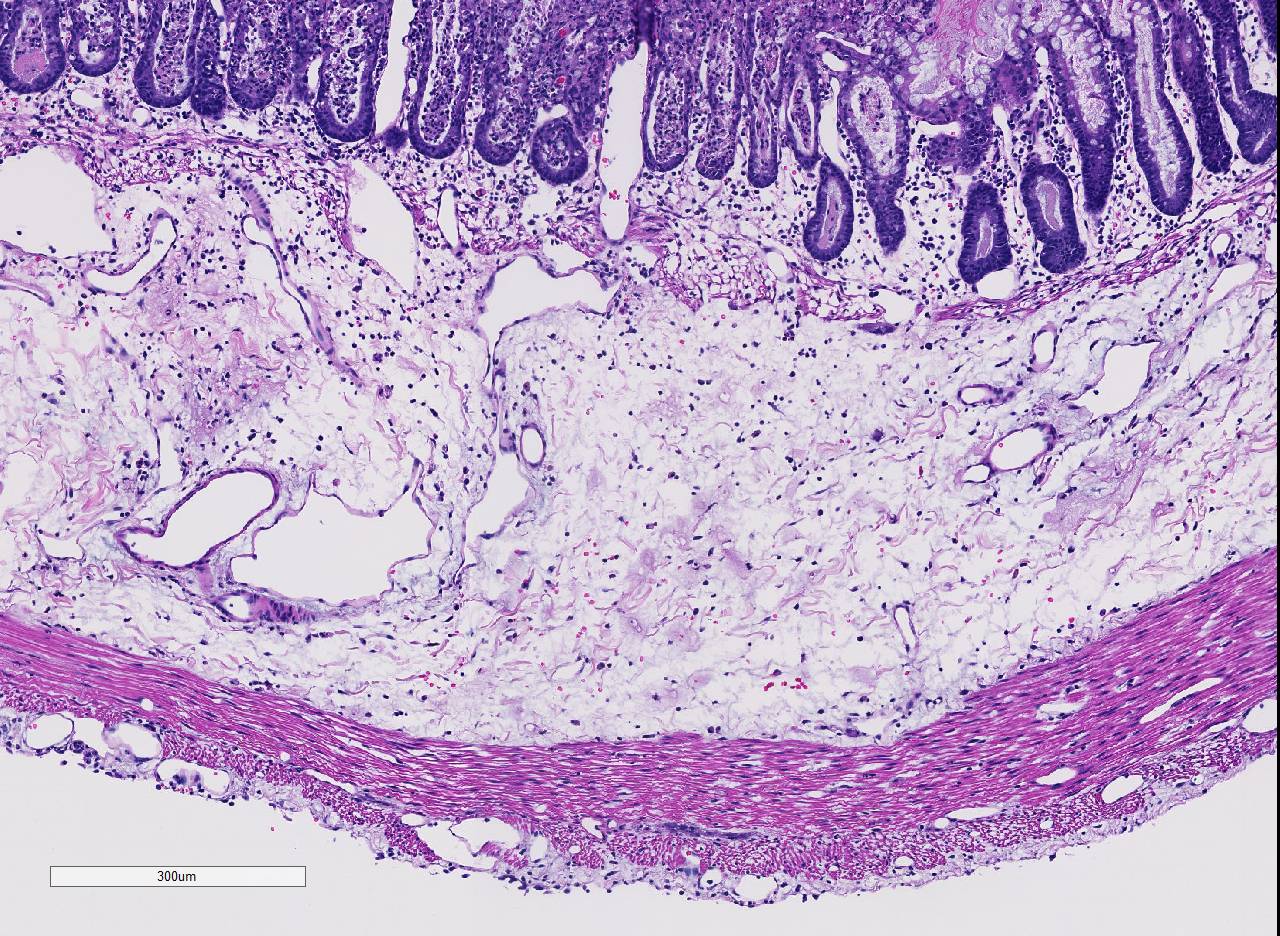
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
C. difficile is a confirmed pathogen in humans and a wide variety of mammals. Infection is a well-recognized clinical entity in horses, pigs, hamsters and rabbits.6 Disease in horses occurs in both adults and foals, with both antibiotic treatment and hospitalization being clear risk factors. Gross and microscopic lesions may not be sufficient to distinguish CDAD from other causes of acute equine enteric disease, including those associated with other clostridial pathogens.3 Conversely, primarily neonatal pigs appear susceptible to infection usually around 5 days of age. 14 Hamsters are the most susceptible animals to natural infection, with as little as 1 colony forming unit leading to disease in antibiotic pretreated animals. 7Although C. spiriforme is a more common pathogen, C. difficile does cause peracute death without clinical signs in rabbits.2
Reports of natural or experimental infection exist in many other species including guinea pigs, mice, rats, domestic cats and dogs, calves, ostriches, prairie dogs and nonhuman primates. 6,12 This is to the contributors awareness, the first documented cases of the entity in this primate species (marmoset).
Although the cecum and colon are the sites most often affected in the majority of species, the nature of lesions present, their location/distribution, and age of host susceptibility all vary significantly. Specifically, foals and rabbits consistently develop severe lesions in the small intestine.6
C. difficile is recognized as one of the most important nosocomial pathogens in humans, causing illness ranging from mild diarrhea to fulminant colitis. Antibiotic use and hospitalization are significant risk factors, with recurrence being common (15-20% of cases). The disease has been well characterized histologically, occurring in three stages: 1) focal epithelial necrosis along with fibrin-rich exudates, 2) marked exudation protruding through an area of mucosal ulceration (characterized as classical volcano lesions), and 3) diffuse, more severe mucosal ulceration with necrosis and associated pseudomembrane composed of fibrin, leukocytes and cellular debris. In cases where disease has progressed to pseudomembrane formation, endoscopy can be diagnostic, and although a rapid means of testing, it is invasive and expensive.10
C. difficile infection occurs when the natural flora in the gut is disrupted. 9 Although this is often associated with antibiotic administration, in some species, stress, change of diet, transportation, starvation, and medical or surgical treatment can initiate disease. 3 After oral ingestion, spores, which are resistant to the acidity of the stomach, germinate into the vegetative form in the small intestine, leading to toxin production resulting in colitis. 4 Other factors such as host susceptibility or virulence factors of the infecting strain may also be integral in determining the clinical outcome of infection. Both toxins, TcdA and TcdB, are cytotoxic, causing disruption of the actin cytoskeleton and tight junctions, resulting in decreased transepithelial resistance, fluid accumulation and destruction of the intestinal epithelium. These substances also cause the release of various inflammatory mediators from enterocytes, mast cells and macrophages. 9 In humans, host response appears critical and protective in the clinical outcome, with patients having elevated levels of IgG and IgA against toxin A.13
The detection of C. difficile by culture is rarely performed for diagnostic purposes because of the slow turnaround time. 5 Rather, identification of C. difficile toxins in the stool using ELISA methods are commonly used. These have excellent specificity, but variable sensitivity (75- 85%). PCR methods to detect the toxin A and B genes responsible for the production of virulence factors are available (Cleveland Clinic paper synopsis).
Although infection and disease in man and animals has long been considered nosocomial and/or antibiotic related, recent studies have explored whether zoonotic and foodborne transmissions occur. Certainly significant percentages of both companion and food animals are known to carry C. difficile. Although there are no scientific reports explicitly confirming that C. difficile can be acquired via foods or contact with animals, there is sufficient laboratory and epidemiological data to suggest that this may occur and that interventions to prevent transmission should be adopted.5,11
The specific source of the infection in these marmosets was not determined, nor was the role of the fluoroquinolone treatment used in their initial empirical therapy (though this class of antimicrobials may pose a greater risk for development of disease than others).4 This case does underscore the importance of awareness by both clinicians and pathologists of the potential for antibioticassociated diarrheal diseases to occur in multiple species.
JPC Diagnosis:
Conference Comment:
Conference participants noted the presence of a prominent circumferential fibrinonecrotic membrane replacing the apical mucosal epithelium, with a sharp line of demarcation separating necrotic tissue from relatively unaffected crypt epithelium. Also, within ulcerated areas this case nicely demonstrates the classic volcano lesions of florid fibrinosuppurative exudation through intestinal crypts.3 As mentioned by the contributor, C. difficile elaborates A and B toxins which diffuse into the tissue from the lumen and destroy the apical mucosal epithelium, in addition to causing the marked submucosal edema demonstrated in this case. Necrosis of the deeper mucosal surface and the colonic tunica muscularis has been reported in chronic cases.3
In addition to C. difficile conference participants discussed measles virus as a unique differential for enteritis in common marmosets, and other new world monkeys. This highly contagious, aerosolized virus belongs to the genus Morbillivirus, in the Paramyxoviridae family.1,8 Marmosets are highly susceptible to measles infection and clinical disease is characterized by severe gastritis and colitis.1 High mortality has been reported in marmosets infected with natural virus from human contact.1 Histologic lesions of measles in marmosets include: epithelial necrosis in the stomach, cecum, and colon, with syncytia, and intracytoplasmic viral inclusion bodies in the mucosal epithelium and the gutassociated lymphoid tissue (GALT).8 Modified live human vaccines against measles have produced active disease in marmosets and are not recommended as a preventative.1,8
References:
2. Carman RJ, Evans RH. Experimental and spontaneous clostridial enteropathies of laboratory and free living lagomorphs. Lab Anim Sci. 1984; 34: 443-452.
3. Diab SS, Rodriguez-Bertos A, Uzal FA. Pathology and diagnostic criteria of Clostridium difficile enteric infection in horses. Vet Pathol. 2013; 50(6):1028-1036.
4. Gould CV, McDonald LC. Benchbedside review: Clostridium difficile colitis. Crit Care. 2008; 12:203.
5. Gould LH, Limbago B. Clostridium difficile in food and domestic animals: a new foodborne pathogen. Clin Infect Dis. 2010; 51(5):577-582.
6. Keel, MK, Songer JG: The comparative pathology of Clostridium difficile-associated disease. Vet Pathol. 2006; 43: 225-240.
7. Larson HE, Borriello SP. Quantitative study of antibioticinduced susceptibility to Clostridium difficile enterocecitis in hamsters. Antimicrob Agents and Chemother. 1990; 34(7): 1348-1353.
8. Lowenstine LJ. Measles virus infection, nonhuman primates. In: Jones TC, Mohr U, Hunt RD, eds., Monographs On Pathology of Laboratory Animals, Nonhuman Primates. Vol 1. Washington, DC:Springer-Verlag, International Life Sciences Institute; 1993:33.
9. Maja R, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009; 7:526-536.
10. Price AB, Davies DR: Pseudomembranous colitis. J Clinc Pathology. 1977; 30:1-12.
11. Rodriguez-Palacios A, Borgmann S, Kline TR, LeJeune JT. Clostridium difficile in foods and animals: history and measures to reduce exposure. Anim Health Res Rev. 2013; 14:11- 29.
12. Rolland RM, Chalifoux LV, Snook SS, Ausman LM, Johnson LD. Five spontaneous deaths associated with Clostridium difficile in a colony of cotton top tamarins (Sanguinus oedipus). Lab Anim Sci. 1997; 47(5):472-6.
13. Warny M, Vaerman JP, Avesani V, Delmee M. Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection. Infect Immun. 1999; 62(2):384389.
14. Waters EH, Orr JP, Clark EG, Schaufele CM: Typhlocolitis caused by Clostridium difficile in suckling piglets. J Vet Diagn Invest. 1998; 20: 104-108.