Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 1
August 22nd, 2018
CASE IV: 15/191 (JPC 4068156).
Signalment: 19 years old sportpony mare (Equus caballus)
History: The pony had a history of eye problems of 2-3 weeks (exact duration not known). It had a corneal ulcer of the left eye that increased in severity, with left sided progressive neurological clinical signs, external ophthalmoplegia, ceased tear production, absent corneal sensitivity, dry mucosal membranes of the left nasal cavity. Keratitis and uveitis were also noted clinically. The neurological clinical examination indication a lesion affecting multiple cranial nerves associated with the ipsilateral fissure orbitalis.
Gross Pathology: In the left eye, there was a focal extensive ulceration of the cornea with yellow discoloration of the central and ventral areas and a narrow rim of intact cornea laterally and dorsally. At the lower limbus, there was severe hyperemia. After removal of the eye, the mandible and the arcus zygomaticus of the maxilla, the orbital fissure was inspected. A few small nodular proliferations, 2-3 mm in diameter, of a light grey firm tissue was detected associated with nerves in the area. Multiple sections was made in the cranium parallel to the optical canal, cutting the canal from the fissura orbitalis to the cranial cavity obliquely in several sections. Nerves within this canal was thickened with a light grey to white firm tissue and, in continuation with this, similar tumor-like tissue was also detected laterally to the infundibulum of the pituitary gland and optical chiasm. The left trigeminal ganglion also appeared grossly to be involved. The same structures on the right side was unaffected.
Laboratory results: None.
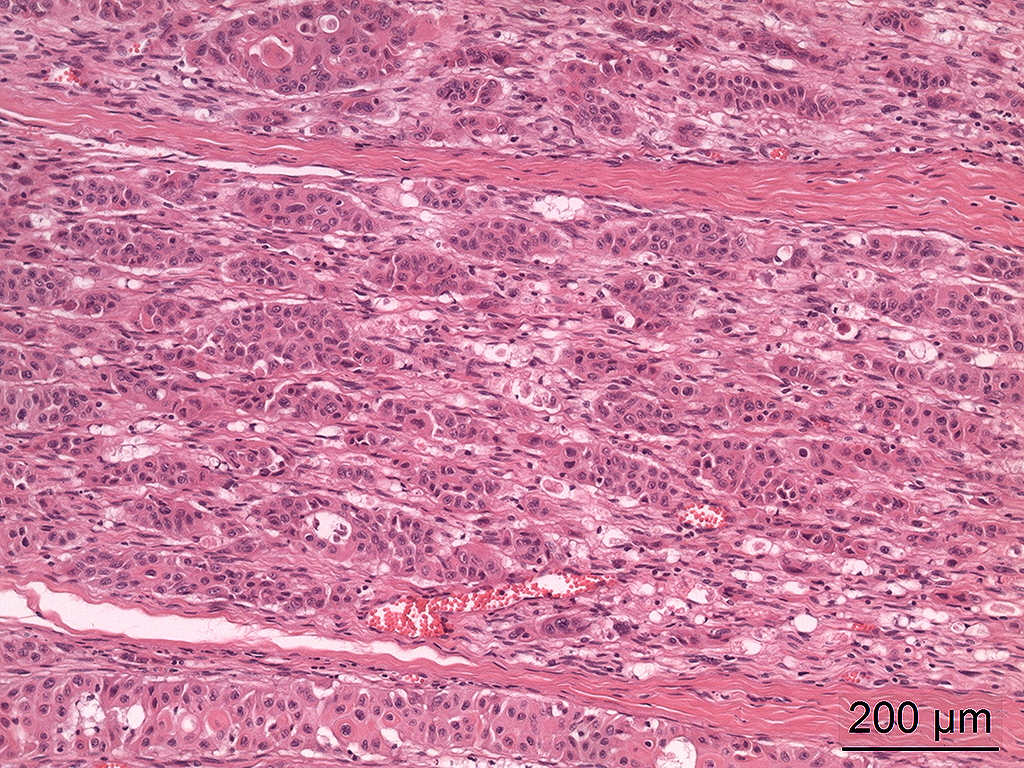
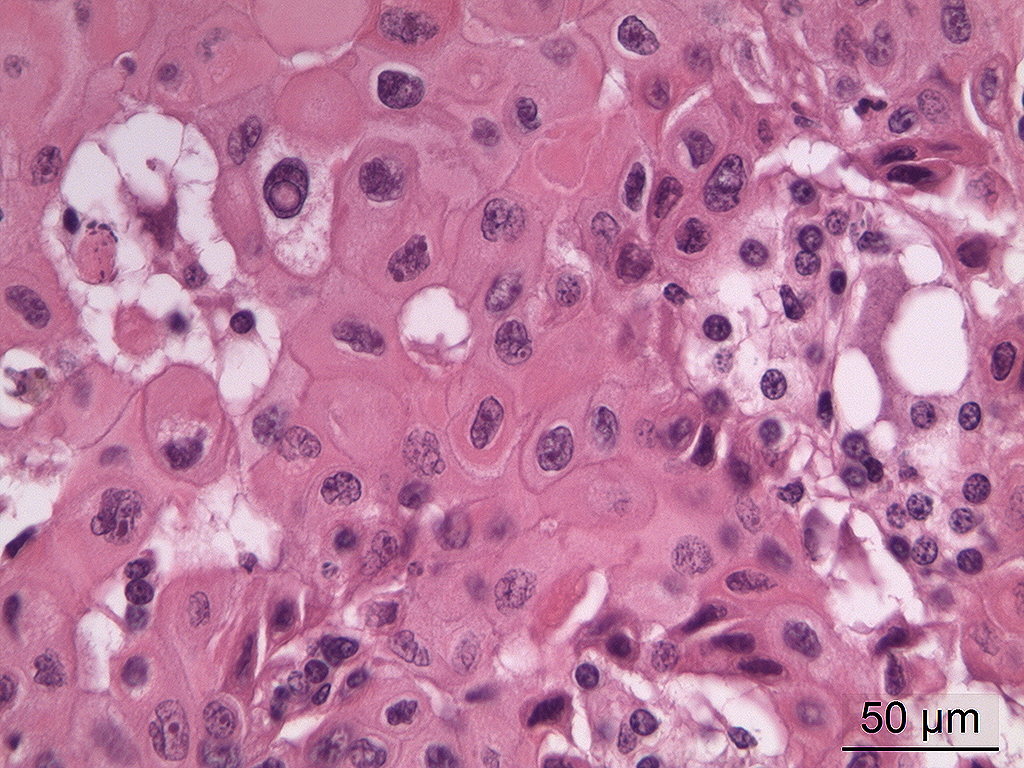
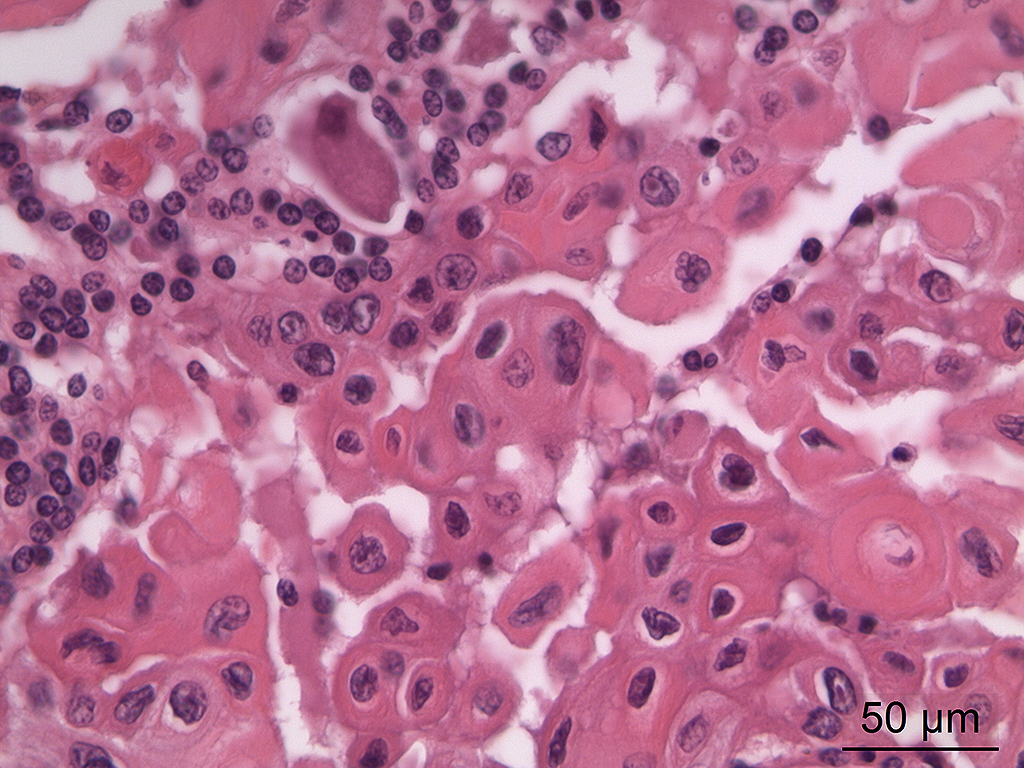
Microscopic Description: In the trigeminal ganglion, the tumor tissue to the left of the pituitary gland, and the above-mentioned cranial nerves, there was a non-encapsulated, infiltrative, cell-rich tumor consisting of squamous epithelial tumor cells growing in islands and trabeculae with moderate amount of stroma. The tumor cells were large with abundant amphophilic to eosinophilic cytoplasm with keratinization of single cells or groups of cells, and large round, oval to irregular nucleus with coarsely stippled chromatin and 1-2 nucleoli with variable size. Anisocytosis and anisokaryosis were prominent, and there was one mitotic figure per 10 40X HPFs. In multiple areas, the tumor cells formed narrow slit-like or larger irregular cyst-like lumens, often with papillary epithelial formations. There was a multifocal mild mononuclear inflammatory cell infiltrate dominated by lymphocytes and multifocal mild mineralization in the stroma. In nerves and in the ganglion remnants surrounding the tumor tissue, there were multifocal degenerative changes.
Contributor’s Morphologic Diagnoses: Cranial nerves and trigeminal ganglion: malignant craniopharyngioma, papillary
Contributor’s Comment: Craniopharyngiomas are rare epithelial tumors of humans and animals arising in the sellar or suprasellar region. They are thought to arise in remnants of craniopharyngeal duct ectoderm that normally forms the Rathke’s pouch,1,4,8 and they may occur in two forms, adamantinomatous and papillary, the former shows morphologic similarities with odontogenic lesions.6 In animals, they usually consist of solid and cystic areas composed of cuboidal, columnar to squamous epithelial cells.1 Craniopharyngiomas are usually described as benign tumors, but some malignant examples of the tumor have been reported in humans and anmials.1,5,6
The tumor in this horse was diagnosed as malignant based on severe pleomorphism and extensive infiltrative growth. Tumors arising in the sellar or suprasellar region may cause different clinical signs depending on which structures they involve; pituitary hormone secretion may be affected either due to compression or involved of the pituitary gland itself, cranial nerve function deficits and CNS dysfunction due to extension into the overlying brain.1 In this horse, the cranial nerve dysfunction was most prominent, and the clinicians suspected that nerves associated with fissura orbitalis was involved. Several cranial nerves enter the lower medial orbita associated with the fissura orbitalis and the foramen rotundum, including the ophthalmic nerve (V1 of trigeminal nerve, cranial nerve no. 5), maxillary nerve (V2 of trigeminal nerve), trochlear nerve (cranial nerve no. 4), oculomotor (cranial nerve no. 3) and abducent nerves (cranial nerve no. 6), however in the horse there is a separate foramen for the trochlear nerve, the foramen trochleare.2,3 Most, if not all, of these nerves were affected by the tumor tissue. The tumor did not exert any pressure on the optic chiasm and the optic nerve was unaffected. The pituitary infundibulum and overlying brain tissue was also unaffected, and in the pituitary gland there was diffuse hypertrophy and hyperplasia of the pars intermedia in addition to a microadenoma of 2 mm in diameter.
JPC Diagnosis: Cranial nerve and ganglion: Craniopharyngioma.
Conference Comment:
Craniopharyngiomas are benign tumors that have primarily been identified in small animals and laboratory species. Their sellar or suprasellar location may make them grossly indistinguishable from other tumors of the hypophysis such as pituitary adenomas, and their growth may result in destruction of the pituitary gland or hypophysis resulting in clinical signs of hypopituitarism or diabetes insipidus. These neoplasms, however, tend to arise in individuals much younger than expected to develop tumors of the pituitary pars intermedia or glandularis.7
Grossly, these neoplasms may advance along the ventral aspect of the brain incorporating and effacing cranial nerves as seen in this case. Extensive growth should not be interpreted as a sign of malignancy.7
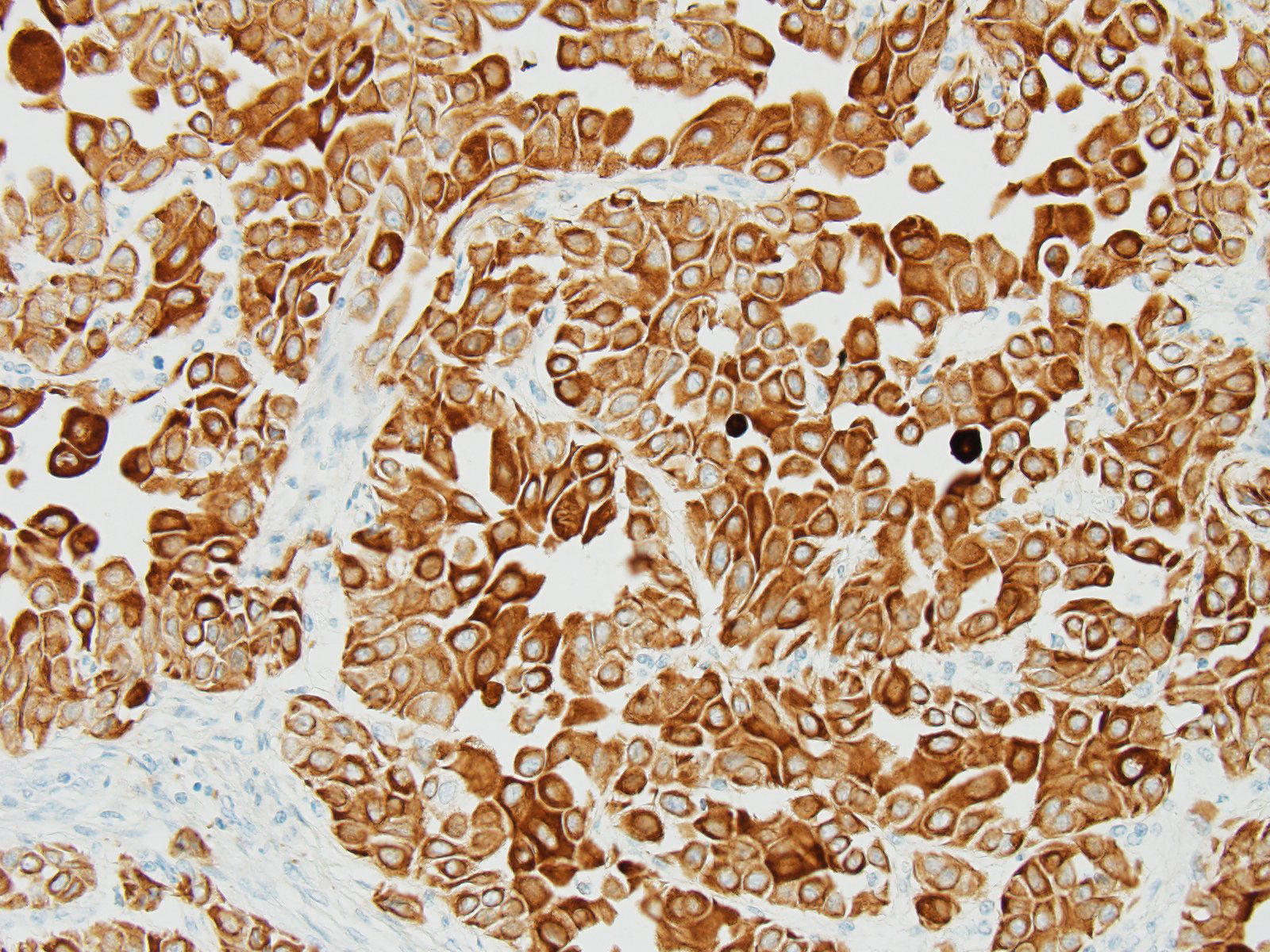
The histological characteristics of this tumor is unique and does not replicate any other neoplasm that may be found in this location of the cranial vault. The distinct features of keratinization results from the ectodermal origin of this tumor as previously discussed by the contributor; and cytokeratin (as performed in this case at the JPC) should be strongly positive. Neoplastic cells are also immunopositive for alpha-fetoprotein suggesting their embryonic level of differentiation; however the AFP stain performed by the JPC was noncontributory, as it is likely not optimized for equine tissues.
Both solid and cystic areas are often seen in these neoplasms.7 Neoplastic cells will occasionally form colloid-like structures containing eosinophilic secretory material (but this was not a significant feature in this case).
Valentine et al.9 has suggested that these tumors be classified as germ cell tumors rather than craniopharyngiomas. In a study of five craniopharyngiomas in young dogs, diagnosis of germ cell tumor is based on 3 criteria: A) a midline suprasellar location, B) the presence of several distinct cell types within the tumor, a germ cell phenotype being one, as well as other cells suggesting differentiation into a secretory or squamous phenotype, and C) positive staining for alpha-fetoprotein.9 The AFP run at the JPC was non-contributory, and neoplastic cells were negative for NSE, GFAP, and S-100. (Interestingly, satellite glial cells were strongly positive for GFAP; enhanced expression of which has been reported by satellite glia in response to nerve injury).
Conference participants discussed potential differentials based on location in the hypothalamus: pituitary adenoma, suprasellar germ cell tumor, and craniopharyngioma. While we strongly considered the contributor’s diagnosis of malignant craniopharyngioma, conference attendees favored a benign neoplasm due to the lack of appreciable pleomorphism or vascular invasion in the distributed sections or any description of metastasis in the submitted history.
Contributing Institution:
Institute of Basic Sciences and Aquatic Medicine
Norwegian University of Life Science, School of Veterinary Medicine
References:
- La Perle KMD. Endocrine system. In: Zachary JF, McGavin MD, ed. Pathologic basis of veterinary disease. 5th St. Louis, USA: 2012: 660-697.
- Liebich HG, König HE. Axial skeleton (skeleton axiale). In: Liebich HG, König HE, eds. Veterinary anatomy of domestic animals. 4th Stuttgart, Germany: Schattauer; 2009:49-112.
- Liebich HG, König HE, Cerveny C. Nervous system (systema nervosum). In: Liebich HG, König HE, eds. Veterinary anatomy of domestic animals. 4th Stuttgart, Germany: Schattauer; 2009:489-560-
- Maxie MG, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s pathology of domestic animals, Vol 1, 5th Philadelphia, USA: Elsevier Saunders; 2007:281-457.
- Pace V, Heider K, Persohn E, Schaetti P. Spontaneous malignant craniopharyngioma in an albino rat. Vet Pathol. 1997;24:146-149.
- Rodriguez FJ, Scheithauer BW, Tsunoda S, Kovacs K, Vidal S, Piepgras DG. The spectrum of malignancy in craniopharyngioma. Am J Surg Pathol. 2007;31:1020-1028.
- Rosol TJ, Meuten DJ. Tumors of the endocrine glands. In: Meuten DJ, ed., Tumors in domestic animals, 5th Ames, IA. Wiley Blackwellpp 70-781.
- Summers BA, Cummings, JF, de Lahunta A. Tumors of the central nervous system. In: Summers BA, ed. Veterinary neuropathology. 1st St. Louis, USA: Mosby-Year Book; 1995:351-401.
- Valentine BA, Summers BA, de Lahunta A. Suprasellar germ cell tumors in the dog: a report of five cases and review of the literature. Acta Neuropathol 1988; 76:94-100.