Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 16
16 January, 2019
CASE II: 1407470-10 (JPC 4050020).
Signalment: 16 year old Male castrated Shetland pony
History: A 16 year old castrated male Shetland pony presented to Oregon State University large animal emergency service for acute onset of respiratory distress. He had an eight-month history of hind end staggering, inappetence, and difficulty maintaining weight.
On physical exam, the gelding was ataxic, dyspneic, tachycardic, tachypneic, and febrile. The complete blood count with unremarkable. Blood gas revealed a high pCO2, bicarbonate, and lactate with low chlorine. Endoscopy revealed bilaterally closed arytenoids on inspiration. Due the history of chronic neurologic disease, plus poor condition, euthanasia was performed and the body was submitted for necropsy.
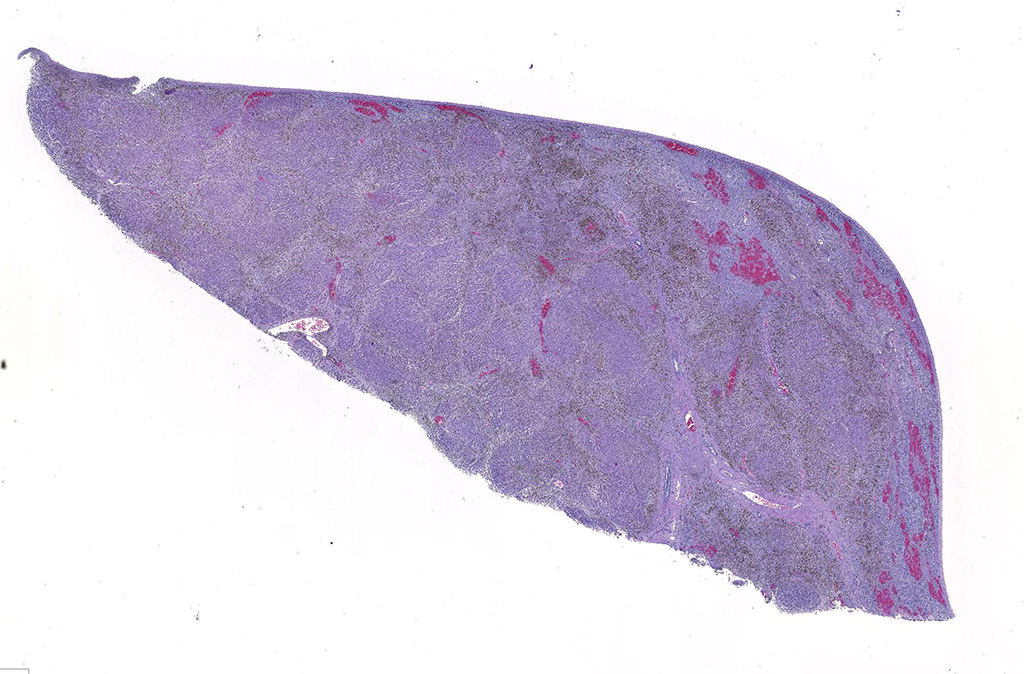
Gross Pathology: At necropsy, the animal was in poor body condition with no subcutaneous, visceral, or pericardial fat. Approximately 200 mL of straw colored fluid was within the thorax and 1 liter of fluid within the abdomen. The liver was markedly enlarged (2x) with swollen, rounded edges, and an irregularly nodular visceral surface. The parenchyma bulged when cut and had a prominent micronodular pattern. The right adrenal gland was swollen by a blood filled 1.5 cm diameter cyst which expanded the medullary area. A few ecchymotic hemorrhages were present on the epiglottis and in the tissue surrounding the trachea. The lungs were diffusely congested and edematous. No significant gross findings were present within the brain, spinal cord, or vertebral column.
Laboratory results: Following histopathology examination a frozen liver specimen was submitted for trace mineral concentration:
|
Mineral |
Horse values |
Reference Range |
Units |
|---|---|---|---|
|
Iron |
27,048 High |
[300-900] |
ug/g |
|
Zinc |
81 Low |
[120-375] |
ug/g |
|
Copper |
25 |
[12-25] |
ug/g |
|
Selenium |
0.5 Low |
[1.0-2.5] |
ug/g |
|
Cobalt |
0.12 Low |
[0.30-0.60] |
ug/g |
|
Molybdenum |
23.7 High |
[1.5-3.0] |
ug/g |
|
Manganese |
9.6 |
[6.0-18.0] |
ug/g |
Frozen serum was retrieved and submitted for ferritin levels at Michigan State University. A limited biochemical profile was analyzed at the OSU VDL.:
|
Mineral |
Horse values |
Reference Range |
Units |
|---|---|---|---|
|
Ferritin |
46,539 High |
[43-261] |
ng/mL |
Liver chemistry profile
|
Analyte |
Result |
Reference Range |
Units |
|---|---|---|---|
|
Bun |
7 Low |
[8-23] |
mg/dL |
|
Total protein |
6.5 |
[5.9-7.6] |
g/dL |
|
Albumin |
3.0 |
[2.9-3.8] |
g/dL |
|
Total bilirubin |
2.9 High |
[0.8-2.6] |
mg/dL |
|
Ck |
3530 High |
[145-633] |
U/L |
|
Alkaline Phosphatase |
742 High |
[80-240] |
U/L |
|
GGT |
430 High |
[7-25] |
U/L |
|
AST (SGOT) |
764 High |
[212-453] |
U/L |
|
SDH |
>170 High |
[2.4-7.2] |
U/L |
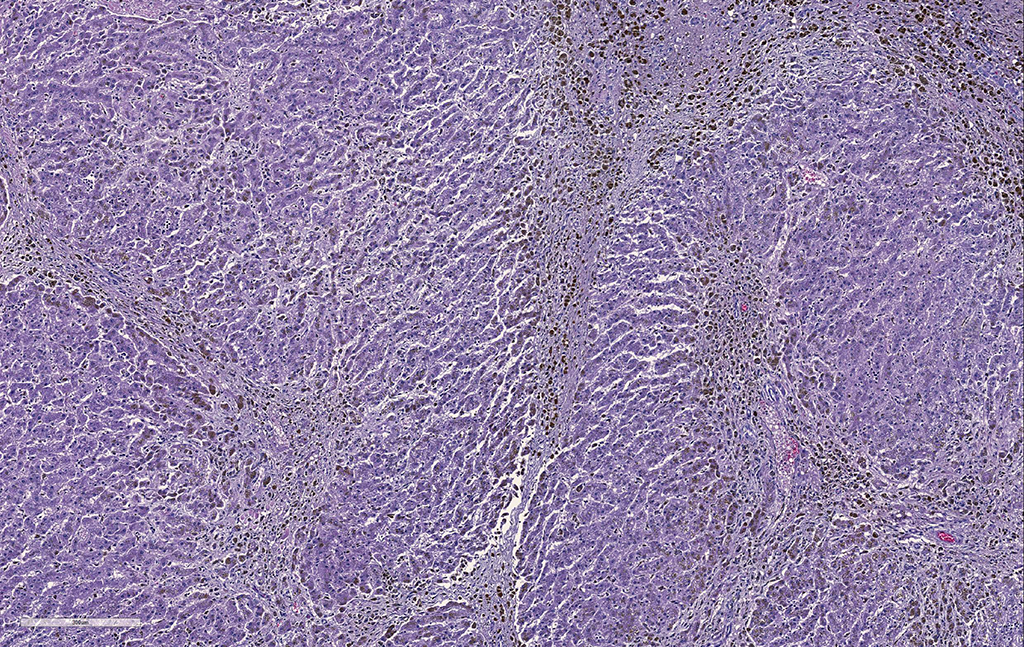
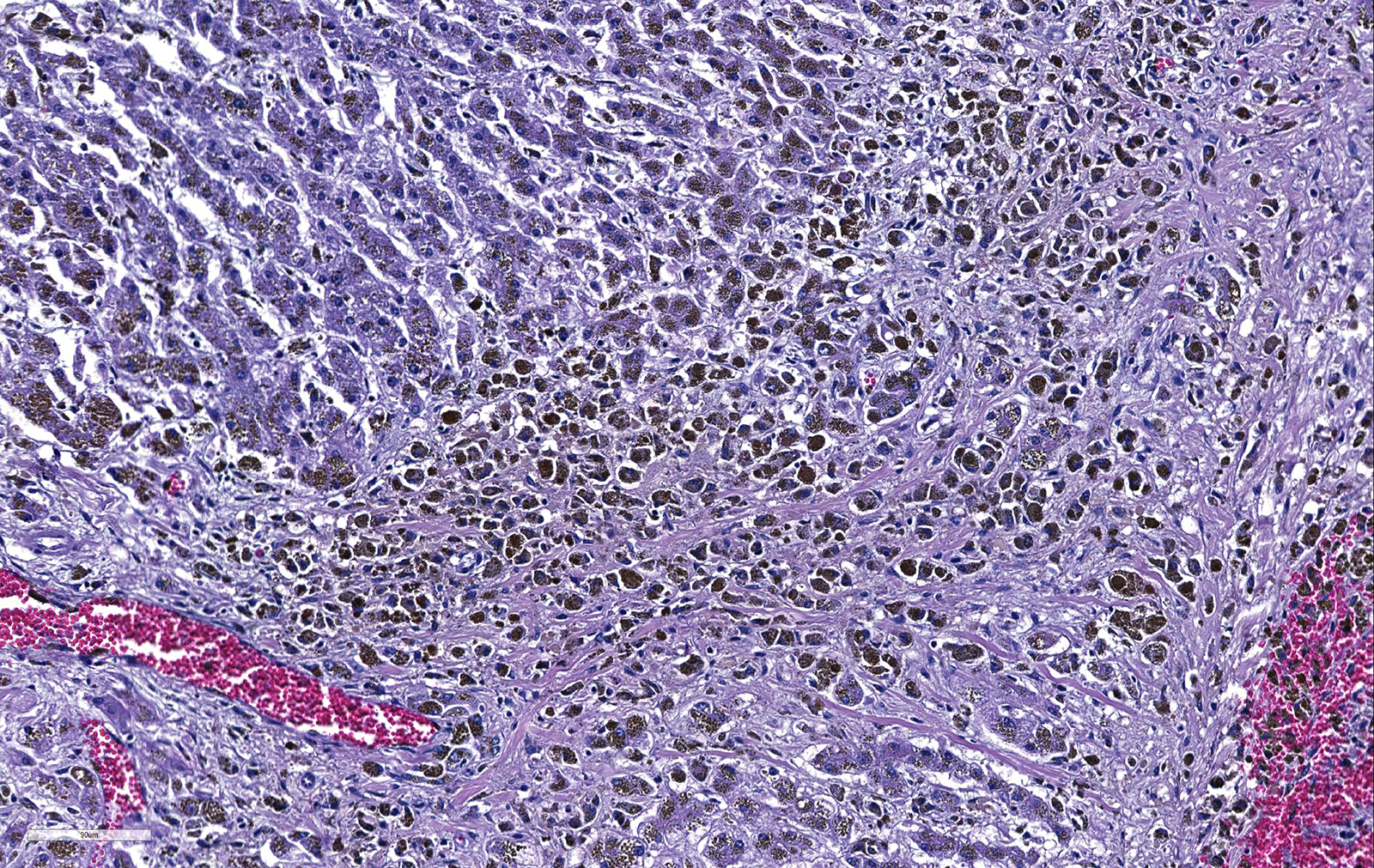
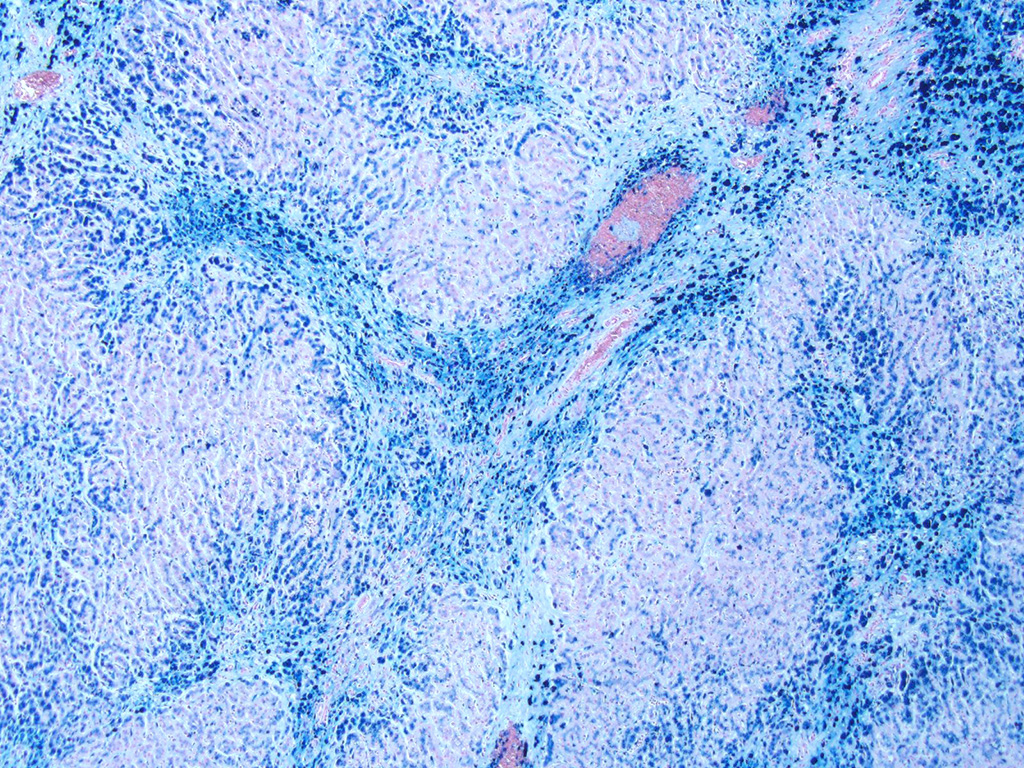
Liver. The hepatic architecture is markedly distorted by severe fibrosis. Pseudolobules are formed from periportal bridging fibrosis with large amounts of collagen in these areas. Along the subcapsular surface, the sinusoidal patterns are disorganized. Infrequently, megalocytes are present but nuclei are generally unremarkable. An abundance of hemosiderin pigment is within stromal macrophages and Kupffer cells while hepatocytes also contain large amounts of iron positive granules. There is moderate cholangiolar hyperplasia.
The thyroid, kidney, pituitary gland, salivary gland, spleen, adrenal gland, pancreas, and colonic epithelial cells as well as mononuclear phagocytes contain the similar brown to golden pigment. Prussian blue staining of the histologic section demonstrated the pigment to be iron.
Cervical, thoracic, lumbar spinal cord (not submitted). All white matter spinal tracts, are vacuolated to some extent, with some vacuoles containing macrophages (digestion chambers). In longitudinal sections, myelin sheaths are disrupted and axons are swollen with spheroid formation.
Cerebrum (not submitted) There are single or 2-3 cell clusters of swollen astrocytes with dispersed chromatin, and scant cytoplasm (Alzheimer type II cells).
Skeletal muscle. Multifocally, the myocytes are hypereosinophilic, with internalization of nuclei and loss of cross-striations.
Contributor’s Morphologic Diagnoses:
Liver: Severe, diffuse hepatocellular degeneration and loss, with bridging portal fibrosis, marked iron accumulation, and mild biliary hyperplasia
Condition: Hemochromatosis
Spinal cord: Moderate myelopathy, consistent with equine degenerative myelopathy
Cerebrum: Mild encephalopathy with Alzheimer type II cells: consistent with hepatic encephalopathy
Skeletal muscle: Mild multifocal myocyte degeneration
Contributor’s Comment: Hemochromatosis is a well described disease affecting humans, fruit-eating and insect-eating birds and in Salers cattle. 2,9,10 In humans, hereditary (primary) hemochromatosis is an autosomal recessive disorder characterized by hyperabsorption of dietary iron and accelerated recycling of iron by macrophages.1 The excessive iron is stored in the parenchymal organs, especially the liver, causing damage which may result in fibrosis, cirrhosis and hepatocellular carcinoma.9 Mynah birds are one of the most frequently reported avian species to have hemochromatosis. Affected livers have marked chronic active cholangiohepatitis with fibrosis and iron granules within hepatocytes, Kupffer cells, and macrophages.8 Iron is also deposited in the other organs. In this present case, Prussian blue stains of histology sections demonstrated excessive iron pigment in the thyroid gland, kidney, pituitary gland, salivary gland, spleen, adrenal glands, pancreas and colon. The serum and hepatic iron levels showed tremendous elevations.
Hemochromatosis patients and mynahs have a defect in the gene encoding for iron proteins (HFE) responsible in the iron sensing mechanism.3,12 The mechanism is well described in humans.10 Dietary iron is used by enteric cells in enzymatic reactions, stored as ferritin, excreted in sloughed enterocytes, or transferred to plasma. Iron absorption is mainly influenced the hepatic protein hepcidin3. It works by blocking ferroportin channels located in enterocytes (and macrophages), preventing iron from reaching the plasma. Dysregulation of this pathway, which is dependent on several genes, can lead to primary hemochromatosis, as low hepcidin levels allow excessive influx and storage of iron3. The mechanism of secondary hemochromatosis is unclear, but occurs in humans as a result of chronic liver disease and hemolytic anemias.15 Untreated, hereditary hemochromatosis patients may develop severe iron overload, whereas in secondary hemochromatosis iron overload is minimal to modest. Also, the pattern of iron accumulation differs in primary and secondary hemochromatosis.5,15 In primary hemochromatosis, iron accumulates within hepatocytes in the periportal region (acinar zone 1) whereas in secondary hemochromatosis iron accumulation is within macrophages and endothelial sinusoidal cells. The pattern observed in this case is more consistent with primary hemochromatosis.2,15 This pony was not on any iron supplements. No other equid in this group has developed liver disease in the year since the diagnosis was made. This suggests that inappropriate intestinal iron absorption as seen with primary hemochromatosis may have been the underlying disease process in this case.
The most common signs of liver disease in horses are non-specific and include icterus, behavioral changes and weight loss.13,15 Histopathology is vital for the definitive diagnosis of hepatic diseases. Potential causes include the acute liver disease known as serum sickness or Theiler’s disease, Tyzzer’s disease, cholangiohepatitis, and hepatic lipidosis.4 Hepatotoxic disease in equids can be separated into pharmaceutical-induced (such as carbon tetrachloride, pentachlorophenols, etc) and plant/environmental exposure (pyrrolizidine alkaloidosis, blue-green algae, Fusarium toxicosis, etc). Iron toxicity has been reported as a cause of hepatotoxicity in orally supplemented foals.10 Biliary calculi (choleliths), liver lobe torsion, and hepatic amyloidosis are other differentials for hepatic disease. Animals with severe destructive hepatic disease may show signs of or acute or chronic hepatic failure (wasting, jaundice) as well as neurological signs.12 Microscopic changes of hepatic encephalopathy may include polymicrocavitation or spongiform changes of white matter (not observed in this case) and Alzheimer type II cells (present in the cerebrum). Spinal cord lesions observed in hepatic encephalopathy include vacuolation at the fasciculus proprius with no histiocytic response. This does not fit with the more widespread microscopic lesions observed in this case.
Excess iron is toxic, causing increased oxidative stress and the production of reactive oxygen species.13 We suspect that excess iron may have promoted oxidative damage, depleting levels of antioxidants such a Vitamin E, leading to the degenerative changes to the myelinated tracks as seen in equine degenerative myelopathy. Acute onset of respiratory distress due to laryngeal and/or pharyngeal paralysis is a proposed, uncommon manifestation of hepatic disease in ponies.13 In this instance, there is a concurrent depression in hepatic selenium levels, and high CK levels, as may be seen with nutritional myopathy, another potential cause of laryngeal malfunction in horses. Sections of heart in this pony had mild multifocal degeneration of cardiomyocytes.
Contributing Institution:
Oregon State University Veterinary Diagnostic Laboratory http://vetmed.oregonstate.edu/diagnostic
JPC Diagnosis: Liver: Fibrosis, bridging and portal, diffuse, severe, with periportal hepatocellular degeneration and loss, edema, biliary hyperplasia and marked hepatocellular and histiocytic siderosis.
JPC Comment: A number of mechanisms are responsible for the excessive storage of iron within the liver in man and many other species of animal. The term hemosiderosis is defined as excessive storage of iron, while the term hemochromatosis, as applied to the liver, indicates a cellular injury resulting from this storage. Primary hemochromatosis is considered to be the result of genetic mutations involving proteins regulating iron metabolism; additional information on the importance of these proteins have been studied in a series of genetic-engineered mutant mice, as well as red deer,6 black rhinoceroses,6 and bottle-nosed dolphins.14
Secondary forms of hemosiderosis result from excessive absorption of iron from sources high in iron, or may result from lack of iron chelators normally found in the diet, which allow iron normal bound in the intestinal lumen, to be freely absorbed (a mechanism which is often commonly identified as a cause of excessive liver iron in a number of zoo and wildlife species in captivity.) Affected zoo species in which hemochromatosis is routinely reported include mynah birds, toucans, birds of paradise, New World monkeys, bottle-nose dolphins and black rhinoceroses.9
A number of studies have been published since the submission of this case to the WSC which have helped to shed light on the pathology and potential pathogeneses of hemochromatosis in a number of species.
A 2018 case report of hemochromatosis from the University of Utrecht16 reported a case of secondary hemochromatosis in 21 horses and one donkey housed together for a minimum of 9 years. Clinical pathology of affected animals revealed blood transferrin saturation levels of >80% and increased gamma-glutamyltransferase levels. Hepatic biopsy demonstrated excessive iron storage in hepatocytes and macrophages, hepatitis and fibrosis. Necropsy of seven animals revealed siderosis in other organs. Excessive levels of iron in drinking water was identified as a the cause, and the remaining 13 animals from 17 to 79 months post diagnosis.
A 2014 study of bottlenose dolphins resulted in the sequence of the hfe gene in this species. The hfe gene is a common cause of primary (hereditary) hemochromatosis in man. The hfe gene’s product regulates the amount of hepcidin produced by hepatocytes. The point mutation of hfe in humans results in improper binding to beta2-macroglobulin preventing the transport of hfe to the hepatocyte surface, where it ultimately upregulates the expression of hepcidin. While a point mutation was not identified in affected dolphins, genomic sequencing is now a tool that may be used to pinpoint hereditary causes of hemochromatosis in animals outside the realm of human and lab animal medicine.14
A recent study of 83 adult Egyptian fruit bats not only documented excessive storage of iron in a number of organs including liver and spleen, but also pancreas, kidney, skeletal muscle, heart, and lung. In addition, 11 animals also had hepatocellular carcinoma, and this represents the first positive correlation between hemochromatosis and hepatocellular carcinoma in a species other than humans.7
References:
- Ajioka R, Kushner J. Clinical consequences of iron overload in hemochromatosis homozygotes. Blood 2003; 3351-3354
- Brown PJ. Haemosiderin deposition in donkey (Equus asinus) livers: comparison of quantitative histochemistry for iron and liver iron content. Res Vet Sci 2011; 284-287
- Coates, T. Physiology and pathophysiology of iron in hemoglobin-associated diseases. Free Radic Biol Med 2014; 1-12
- Cullen J. Liver and biliary system. In: Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, 5th ed. Philadelphia, PA: Elsevier; 2007.
- Klopfleisch R, Olias P. The pathology of comparative animal models of human haemochromatosis. J Comp Path 2012: 147:460-478.
- Lavoie JP, Teushcher E: Massive iron overload and liver fibrosis resembling haemochromatosis in a racing pony. Eq Vet J 1993; (6) 552-554.
- Leone AM, Crawshaw GJ, Garner MM, Frasca S, Stasiak I, Rose K, Neal D, Farina LL. A retrospective study of the lesions associated with iron storage disease in captive Egyptian fruit bats. J Zoo Wildl Med 2016; 47(1):45-55.
- Mete A, Hendriks HG, Klaren PHM, Dorrestein GM, van Dijk JE, Marx JJM. Iron metabolism in mynah birds (Gracula religiosa) resembles human hereditary haemochromatosis. Avian Path 2003; 625-632
- Mullaney TP, Brown CM. Iron toxicity in neonatal foals. Eq Vet J 1988: (20):119.
- O'Toole D, Kelly EJ, McAllister MM, Layton AW, Norrdin RW, Russell WC, Saeb-Parsy K, Walker WP: Hepatic failure and hemochromatosis of Salers and Salers-cross cattle. Vet Pathol 2001: 372–389.
- Pearson E, Hedstrom R, Poppenga R. Hepatic cirrhosis and hemochromatosis in three horses. JAVMA 1994; 1053-1056
- Pearson, EG. Liver failure attributable to pyrrolizidine alkaloid toxicosis and associated with inspiratory dyspnea in ponies: three cases. JAVMA 1991; 198:1651-1654.
- Pearson, EG. Liver disease in the mature horse. Eq Vet Educ 1999; 11:(2) 87-96
- Phillips BE, Venn-Watxon S, Archer LL, Nollens HH, Wellehan JFX. Preliminary investigation of bottlenose dolphins (Tursiops truncates) for hfe gene-related hemochromatosis. J Wildl Dis; 2014; 50(4):891-895.
- Sebastiani G, Walker A: HFE gene in primary and secondary hepatic iron overload. World J of Gastroent 2007; 4673-4689
- Theelen MJP, Beukers M, Grinwis GCM, Oldruitenborgh-Oosterbaan MM. Chornic iron overload causing haemochromatosis and hepatopathy in 21 horses and one donkey. Eq Vet J 2018; epub doi: 10.1111/evj.13029.