CASE II: P18-3879 (JPC 4118085)
Signalment:
8 month-old, female, St. Bernard, Canis lupus familiaris, dog
History:
The patient was presented due to myoclonus of temporal muscles, melena and purulent discharge from the nostrils. It began with depression and anorexia. It also had a non-regenerative anemia and thrombocytopenia that progressed from mild to severe.
Gross Pathology:
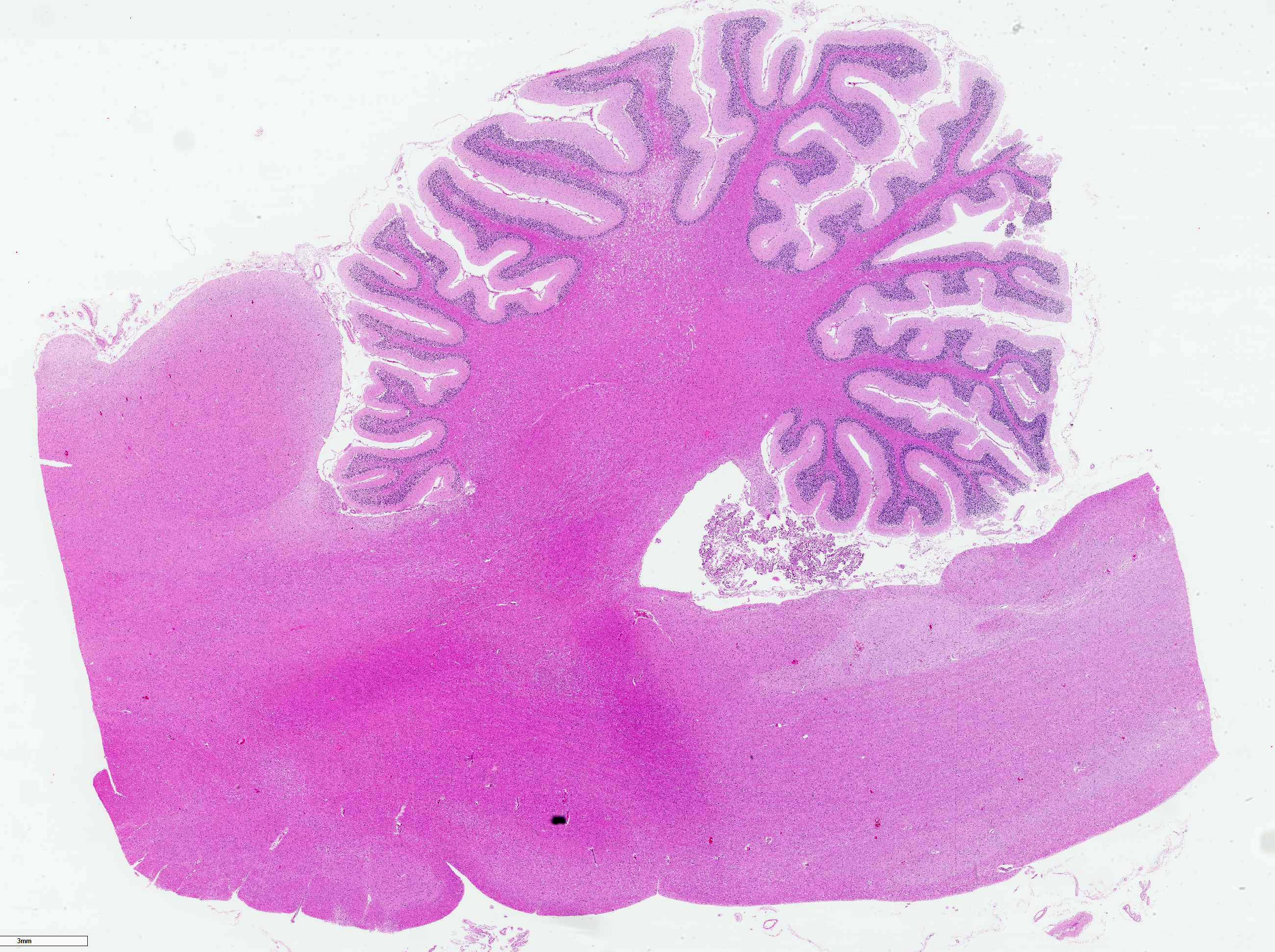
On necropsy, the animal was in good body condition. Lungs were diffusely congested and moderately edematous. There were no gross lesions noted on examination of the brain or spinal cord.
Laboratory results:
Immunohistochemistry (IHC) revealed moderate to strong positive immunoreactivity for canine distemper virus (CDV) in astrocyte nuclei and multifocally in the neuropil next to demyelinated areas.
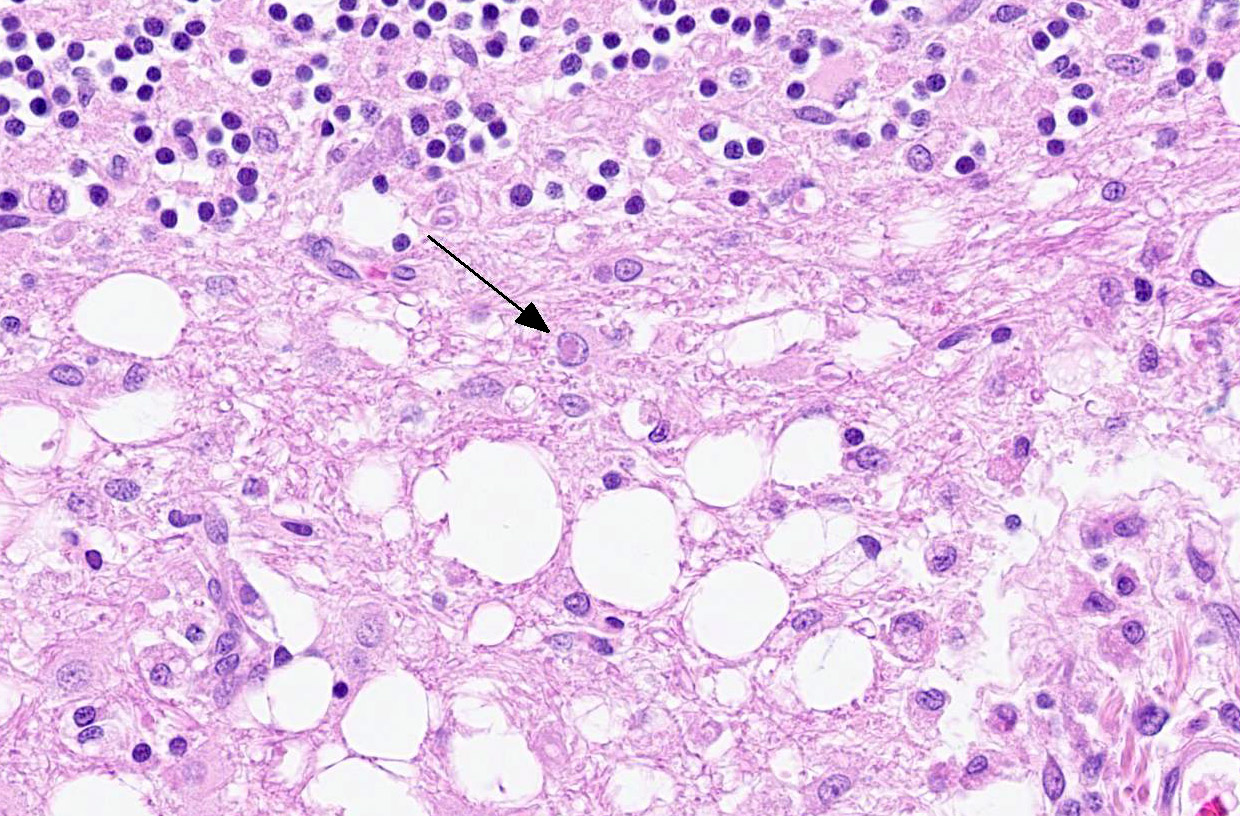
Microscopic Description:
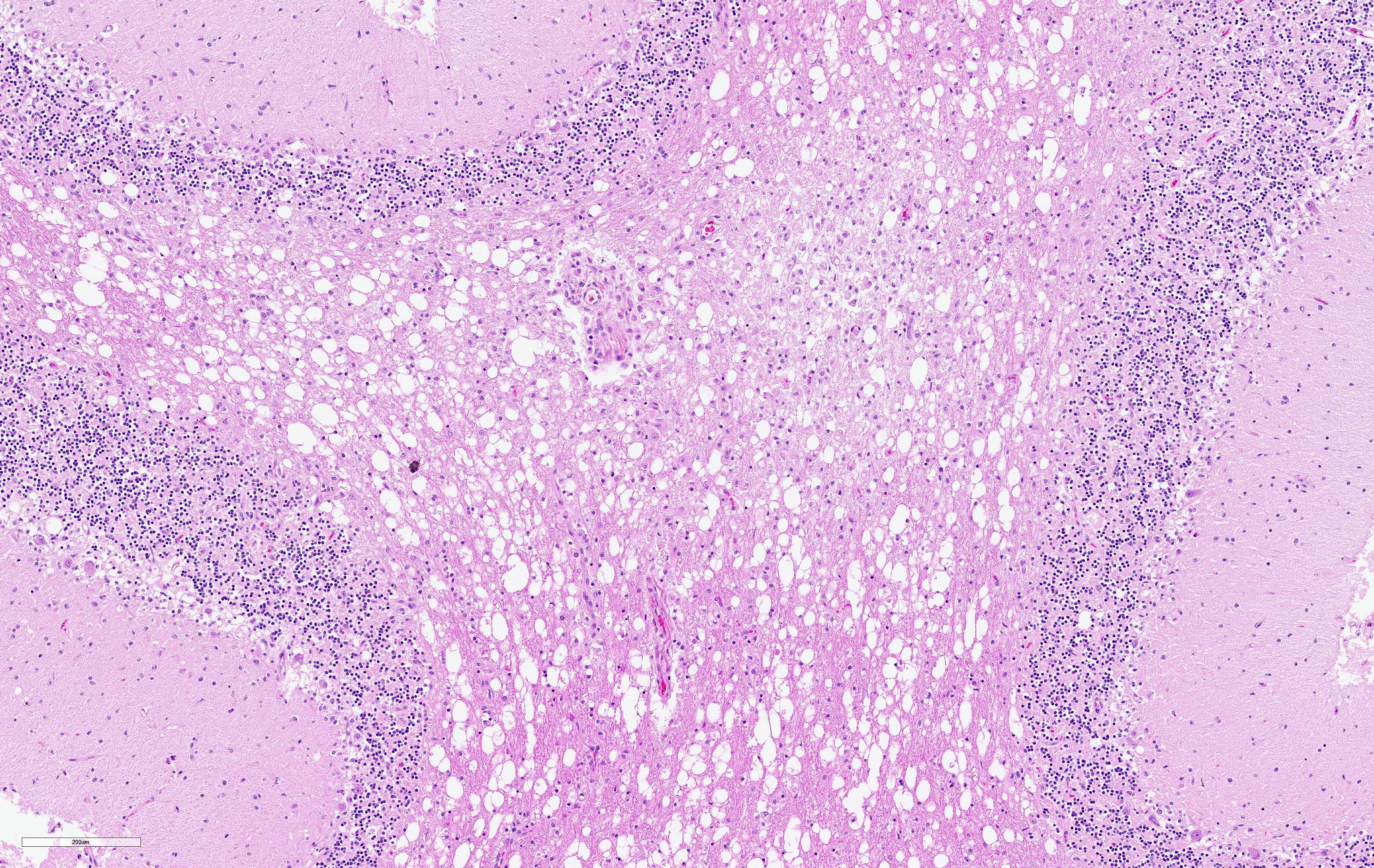
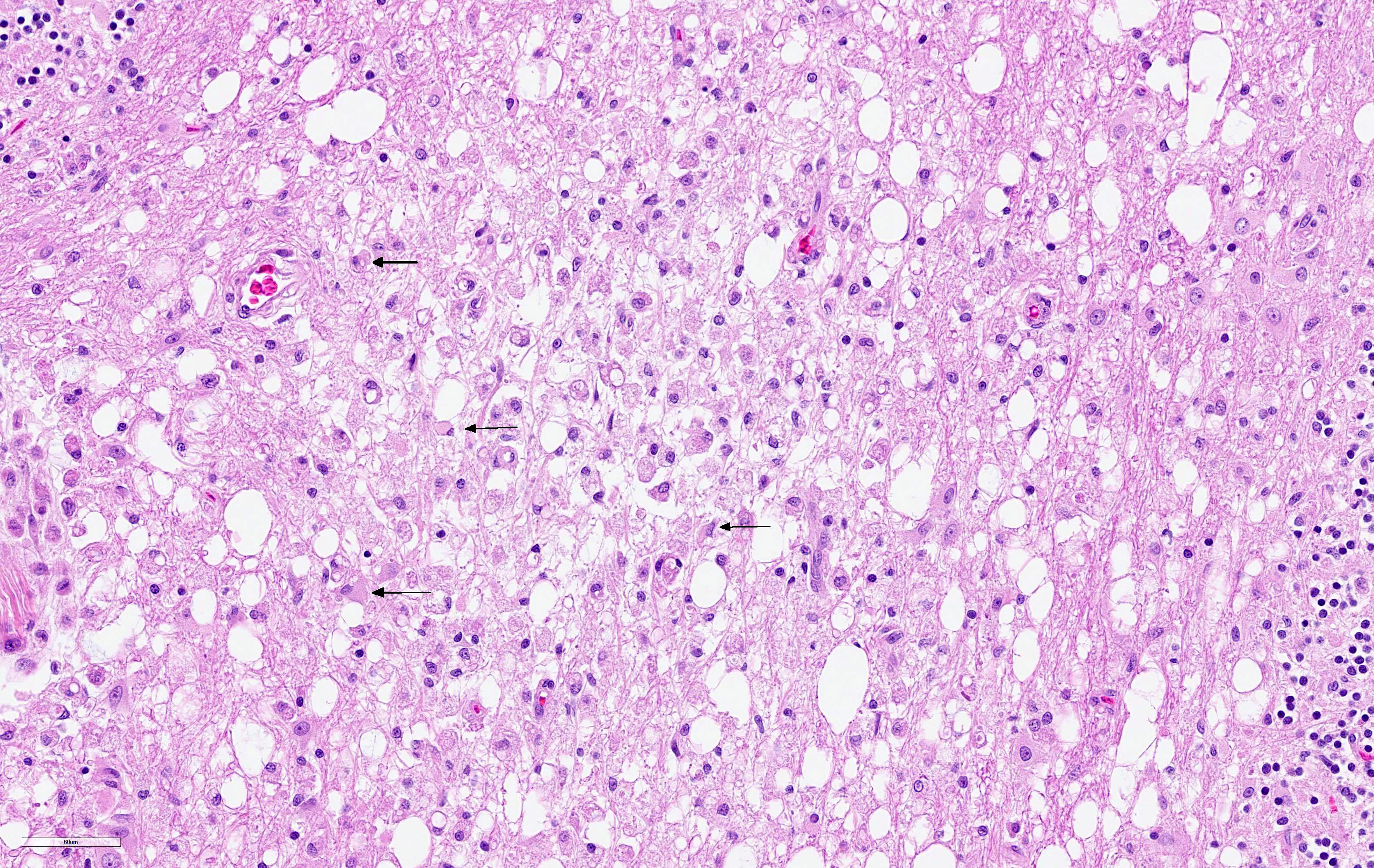
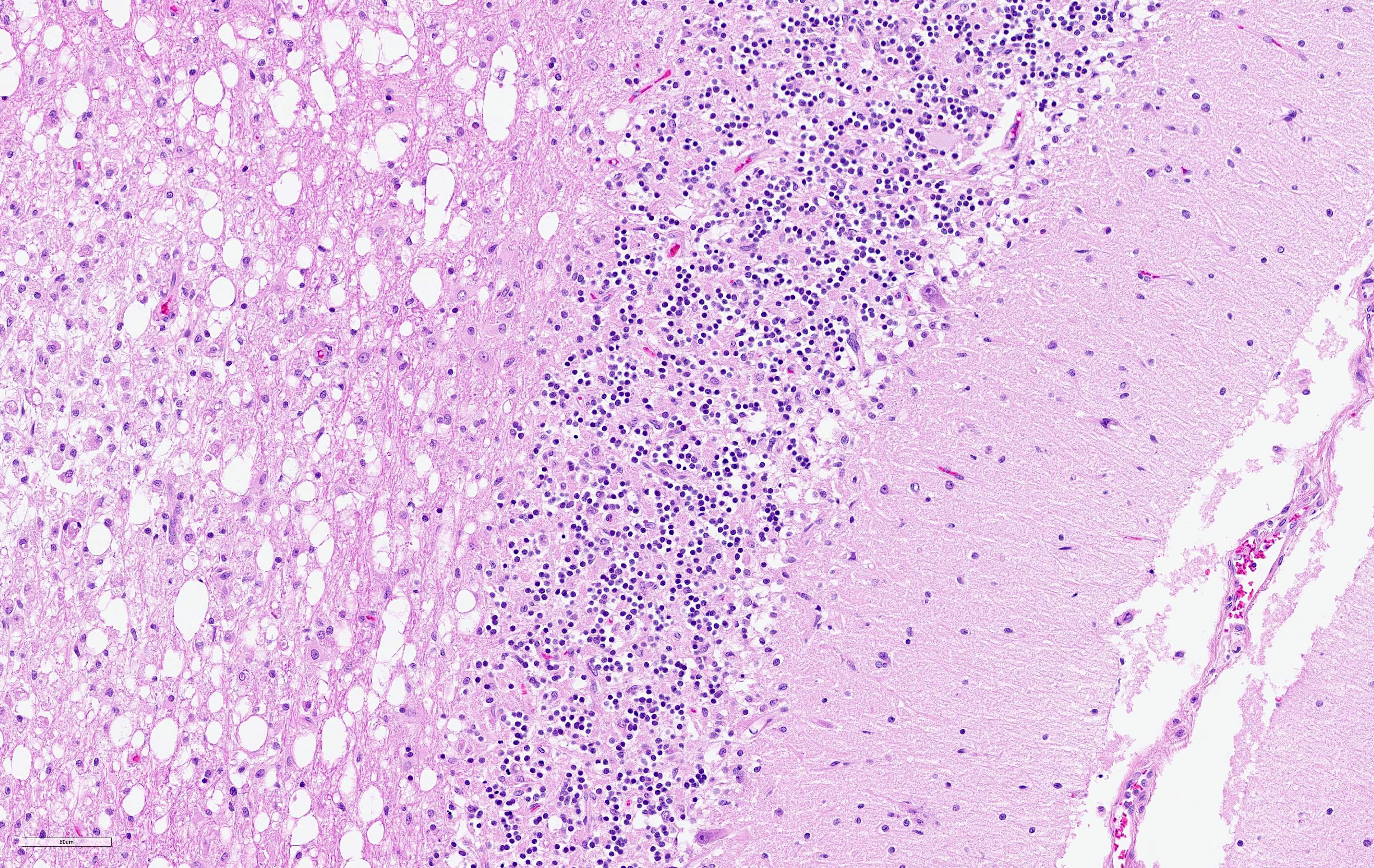
The cerebellar white matter is severely affected by loss of tissue substance and delineated by numerous reactive astrocytes admixed with large numbers of macrophages that contain abundant, intracytoplasmic, degenerate myelin (Gitter cells) and few lymphocytes. There are multiple foci of gliosis in the adjacent neuropil. There are large numbers of astrocytes with large, swollen nuclei that contain a single, round, amphophilic, glassy inclusion. Blood vessels throughout the lesions are reactive and branched and typically are lined by reactive, hypertrophied endothelium.
Contributor's Morphologic Diagnoses:
Cerebellum, white matter: Demyelination and necrosis, severe,
multifocal, with numerous Gitter cells and intranuclear inclusion bodies in
astrocytes.
Contributor's Comment:
The clinical history and the distinctive histologic feature of extensive demyelination with intranuclear inclusion bodies in astrocytes are consistent with canine Distemper in this dog. Canine distemper is caused by a morbillivirus (family Paramyxoviridae), and affects a wide range of terrestrial carnivores, including domestic dogs, wild canids (e.g. wolves, foxes), mustelids (e.g. ferrets, minks), procyonids (e.g. raccoons), ursids (e.g. black bears, giant pandas), ailurids (e.g. red pandas), viverrids (e.g. civets, genets), hyaenids (e.g. spotted hyenas), large felids (e.g. lions, tigers), and marine mammals, where several epidemics with mass mortalities have been reported. Domestic, feral and even wild canids are considered vectors for this infectious agent in wildlife species.3
Canine Distemper Virus (CDV) is shed in secretions mostly from the respiratory tract and infection is usually acquired by inhaling aerosols or by close contact with infected dogs. The virus infects monocytes and macrophages of the upper respiratory tract, which convey it to tonsils and local lymph nodes during the first 24 hours. The virus replicates further in local lymphoid tissues, and by 2-5 days after exposure is present in lymphoid tissues throughout the body, including thymus, spleen, bone marrow, and intestinal lymphoid tissue. During the first viremic phase, generalized infection of lymphoid tissues with lymphoid depletion, lymphopenia and transient fever is observed. Severe immunosuppression is a consequence of leukocyte necrosis, apoptosis and dysfunction. Gross lesions include lymph node swelling, depletion of MALT and reduced thymus size. Lesions are microscopically characterized by a generalized depletion of T and B cell compartments in spleen, lymph nodes, MALT and tonsils as well as hyperplasia of reticular cells in the medullary region of lymph nodes. A second viremia provokes high fever and infection of parenchymal tissues such as the respiratory tract, digestive tract, skin, and CNS. Development of the disease is highly dependent on the immune status of the host, the titer of antibodies to envelope glycoproteins, the age of the host, and the strain of virus. The infection is systemic, and clinical signs usually involve the respiratory, gastrointestinal, and nervous systems.2,3,4
The main respiratory lesion is bronchointerstitial pneumonia, with formation of inclusion bodies in the cytoplasm of bronchial and bronchiolar epithelial cells, type II pneumocytes and alveolar macrophages. Alveolar epithelial syncytial cells are a characteristic feature when present. Enteral infection leads to catarrhal enteritis with depletion of Peyer's patches. Often enteric and respiratory lesions are worsened by secondary bacterial infections. Characteristic dermal manifestations include hyperkeratosis of foodpads and nasal planum (hard pad disease), and pustular dermatitis (distemper exanthema). In young animals, enamel hypoplasia and metaphyseal osteosclerosis (persistence of the primary spongiosa in the metaphyses of long bones), can be seen following CDV infection. Neurologic signs depend on viral distribution in the CNS and include cerebellar and vestibular signs, hyperesthesia, seizures, cervical rigidity, as well as paraparesis or tetraparesis with sensory ataxia. Histological manifestations include polioencephalitis and demyelinating leukoencephalomyelitis. Demyelination in white matter tracts is most severe in the cerebellum, rostral medullary velum, optic tracts, spinal cord, and surrounding the fourth ventricle, and probably arises from distribution of virus through the cerebrospinal fluid. Astrocytes usually contain nuclear and, rarely, cytoplasmic inclusions.2,3,4
CDV may enter the brain via infected mononuclear cells trafficking through the blood-brain-barrier, which results in local virus release and subsequent infection of resident epithelial and endothelial cells. Choroid plexus cells and brain vessels cells are firstly infected. Once inside the brain, the virus spreads via the cerebrospinal fluid (CSF), where it may infect ependymal lining cells of the ventricles and ultimately glial cells and neurons. Astrocytes are the main cell population infected by CDV, whereas neuronal infection is more prominent during the early phase and most prominent in distemper polioencephalitis. Oligodendroglial infection by CDV has been inconsistently documented.5
There are two recognized stages in the development of CDV-induced demyelination. In acute demyelinating lesions, there is massive down-regulation of myelin transcription and metabolic impairment of the myelin-producing cells, but there is no evidence that oligodendrocytes are undergoing apoptosis or necrosis. It has been suggested that CDV induced microglial cell activation could contribute to oligodendrocyte/myelin damage. Although perivascular inflammation is entirely lacking, some CD8+ cells are found in acute demyelinating lesions. The invasion of these cells might be triggered by microglial cell activation. Metalloproteinases and their inhibitors also appear to be strongly upregulated in the acute stage. Six to seven weeks post-infection, perivascular inflammation with lymphocytes, plasma cells and monocytes is detected. This inflammation, together with the demyelinating lesions leads to progression of tissue damage. Pro-inflammatory cytokines are markedly upregulated, whereas anti-inflammatory cytokines remain at normal levels. Chronic inflammatory demyelination is due to a bystander mechanism resulting from interactions between macrophages and antiviral antibodies.7
Contributing Institution:
Departamento de Patolog?a (Pathology Department). Facultad de Medicina Veterinaria y Zootecnia, Universidad Nacional Aut?noma de M?xico. Mexico City, Mexico. http://fmvz.unam.mx/fmvz/departamentos/patologia/acerca.html
JPC Diagnosis:
Cerebellum and brainstem: Demyelination, multifocal to coalescing, moderate, with gliosis and astrocytic intranuclear inclusions.
JPC Comment:
The contributor provides an excellent thorough explanation of the pathogenesis and variety of lesions associated with canine distemper virus (CDV), a morbillivirus that infects numerous domestic and wildlife species.
A novel strain of CDV named "American-4" was recently identified following an increased number of cases of CDV that were seen at the University of Tennessee, including vaccinated dogs. A subsequent study found 77% of CDV positive raccoons and gray foxes in Tennessee to be infected with this strain.8 Another study found serum antibody titer responses to this strain did not increase following vaccination in dogs despite increased responses to the vaccine strain. Cross-protection was titer-dependent with higher titers required for adequate protection, suggesting decreased cross-protection against this emergent strain, likely as a result of antigenic strain differences.7
Domestic species are important reservoirs of CDV throughout the world and can be a source of wildlife exposure to CDV. Wildlife species in turn are also frequently implicated as the source of CDV outbreaks in both domestic and non-domestic species, and often serve as intermediate hosts of CDV.8
One such outbreak occurred in 2016 at a private east Tennessee zoo, resulting in the first pathologic description of the virus in sloths. Five of eight wild caught 2-toed sloths (Choloepus didactylus) died while completing a 15-month quarantine following a 2-3 week history of hyporexia, lethargy, and oronasal discharge. Common gross findings included multifocal crusts and ulcers within the oral cavity, lips, and nares, which corresponded to discrete eosinophilic intranuclear and intracytoplasmic inclusion bodies in epithelial cells observed during microscopic examination. In addition, neutrophilic, necrotizing, bronchointerstitial pneumonia was present in all cases with syncytial cells seen within the bronchiolar epithelium.8
A striking feature noted in this brief report was that each animal was also affected by hepatic necrosis with numerous viral inclusions observed within hepatocytes, which is not typically seen in domestic canines and nondomestic carnivores.8
No sloths had gross or histologic lesions in the central nervous system (CNS); however, CDV antigen was found to be present within meningeal vessels, choroid plexus, and ependyma. It is possible no CNS lesions were observed given that all affected animals had severe systemic disease and insufficient time may have elapsed prior to death for their development.8
Virus isolation and sequencing of tissue from one affected sloth determined it was infected with the American-4 strain of CDV. Although the sloths were housed indoors, it is possible transmission occurred as the result of exposure to organic material or other fomites exposed to wildlife.8
Interestingly, three of four adult kinkajous (Potos flavus) housed in the same building died after developing similar clinical signs. CDV was confirmed via polymerase chain reaction (PCR) in one animal. Similar to the sloths, none had CNS lesions and one had hepatic necrosis and intranuclear inclusions in hepatocytes. Virus isolation sequencing was not performed although the most likely etiology in this case was also the American-4 strain of CDV. 8
Conference participants observed and discussed the loss of Purkinje and up to 50% of granular cells in areas adjacent to folial demyelination. This finding is not classically described in regard to CDV. It is possible these cells were lost due to the virus' tropism for astrocytes and subsequent changes in the neuropil microenvironment. Astrocytes perform many roles within the CNS, including regulation of ionic exchanges between cells and the formation of functional connections by production of molecules that are tropic for other specialized cells of the CNS.1
References:
1. Cantile C, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 6th ed. Vol 1. Elsevier, St. Louis, Missouri; 2016:260-262.
2. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy and Palmer's Pathology of Domestic Animals. Vol 2. 6th ed. St. Louis, MO: Elsevier limited; 2016:574-576.
3. Beineke A, Baumg?rtner W, Wohlsein P. Cross-species transmission of canine distemper virus?an update. One Health. 2015; 1:49?59
4. Beineke A, Puff C, Seehusen F, Baumg?rtner W. Review paper: Pathogenesis and immunopathology of systemic and nervous canine Distemper. Veterinary Immunology and Immunopathology. 2009; 127: 1?18
5. Lempp C, Spitzbarth I, Puff C, Cana A, Kegler K, Techangamsuwan S, Baumg?rtner W, Seehusen F. Review: New Aspects of the Pathogenesis of Canine Distemper Leukoencephalitis. Viruses. 2014; 6:2571-2601.
6. Stilwell JM, Anis E, Wilkes RP, Rissi DR. Dual infection with an emergent strain of canine distemper virus and canine parvovirus in an Arctic wolf under managed care. J Vet Diagn Invest. 2019;31(4):594-597.
7. Vandevelde M, Zurbriggen A. Demyelination in canine distemper virus infection: a review. Acta Neuropathol. 2005; 109: 56?68.
8. Watson AM, Cushing AC, Sheldon JD, et al. Natural Canine Distemper Virus Infection in Linnaeus's 2-Toed Sloths (Choloepus didactylus). Vet Pathol. 2020;57(2):311-315.