Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 17
January 31st, 2018
CASE I: 186 (JPC 4079216).
Signalment: Adult, freshwater crocodile, Crocodylus johnsoni, reptile.
History: Found dead. No preceding clinical signs.
Gross Pathology: None
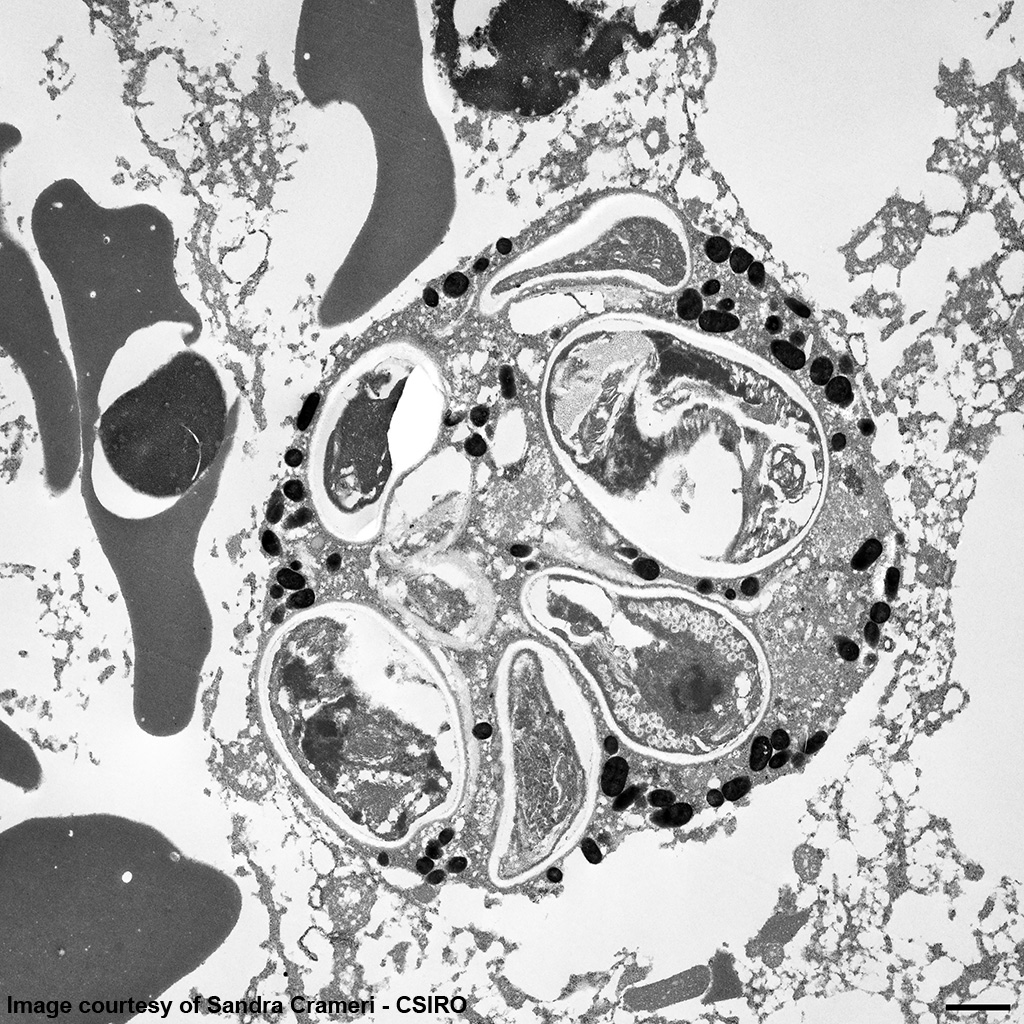
EM Image Description:
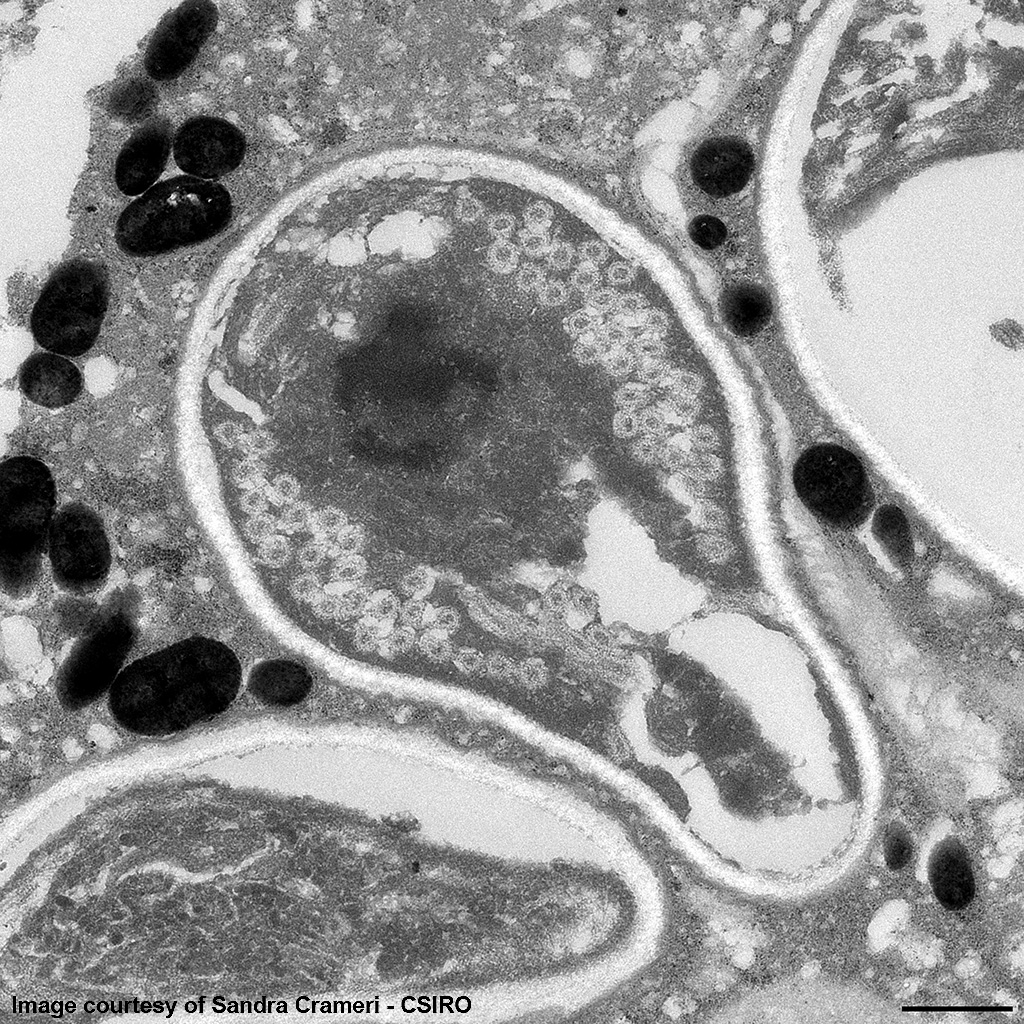
The image shows multiple intact nucleated red blood cells and centrally a cell with a parasitopherous vacuole containing numerous spores. The higher magnification image of a single spore shows it to have a thick, multilayered wall composed of an electron-dense outer layer (exospore) an electron-lucent inner layer (endospore) and a plasma membrane enclosing the cytoplasm. At the posterior end of the cytoplasm there is a distinct nucleus to the center, a poorly defined posterior vacuole and multiple coils of the polar filament to the periphery. At the anterior end there is an anchoring disc underlying which are the membranes of the polaroplast. The anchoring disc is connected to the polar filament.
Contributor’s Morphologic Diagnosis: Intrahepatic microsporidiosis
Molecular diagnostics:
Sequencing of product amplified by Universal Microsporidian primers produced a 96% match across 276 base pairs to Encephalitozoon hellem (Animal Health Laboratories, Department of Agriculture and Food, Western Australia).
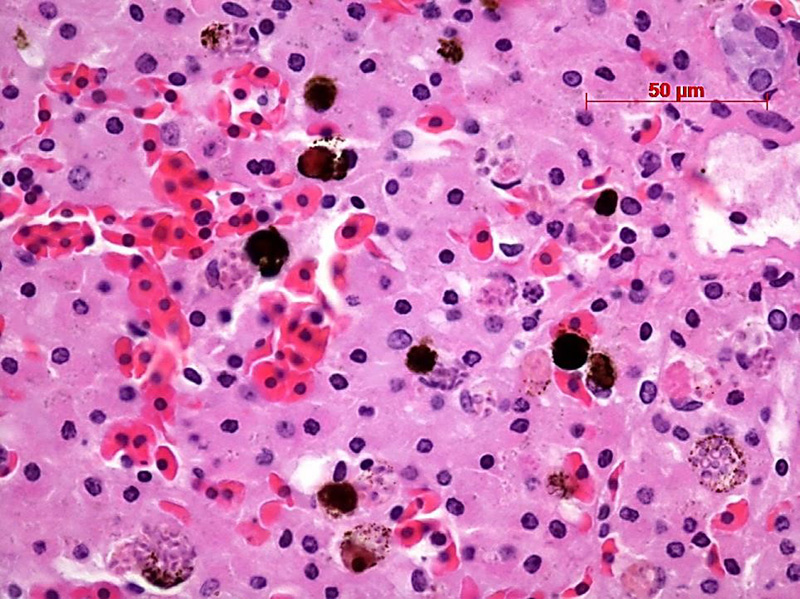
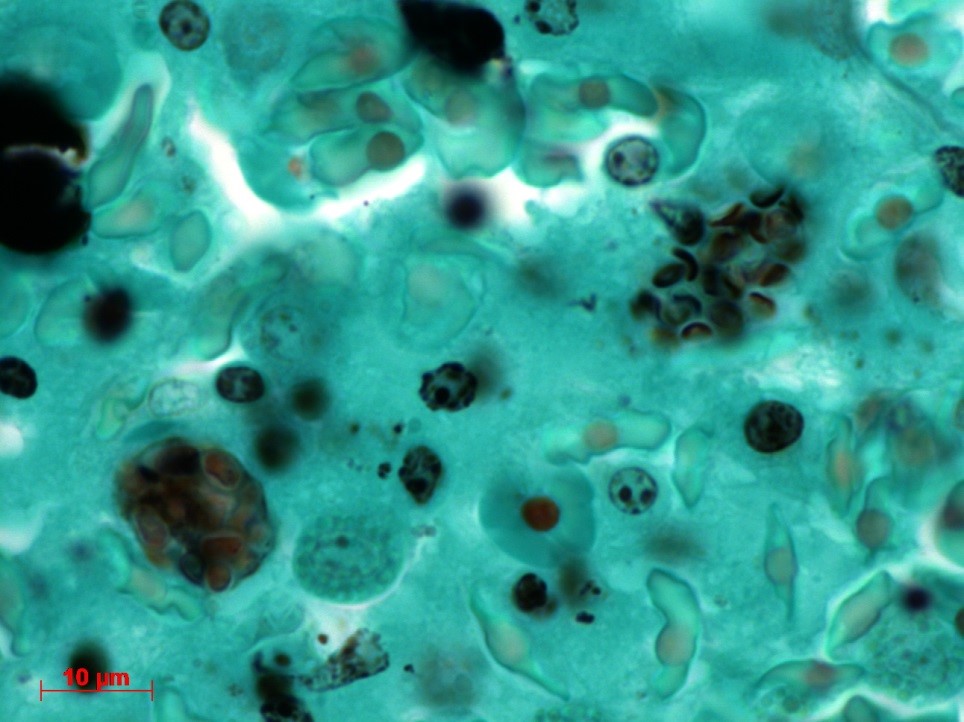
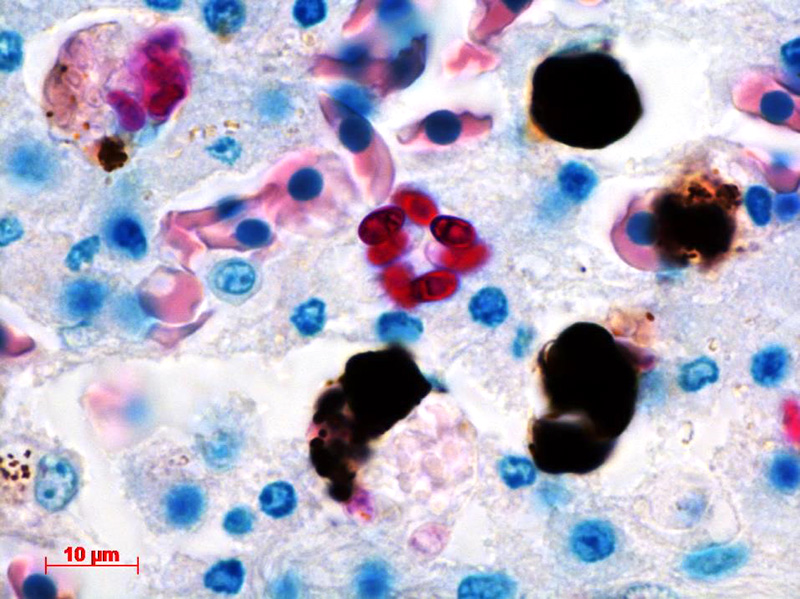
Contributor’s Comment: The ultrastructural features of this organism are consistent with mature spores of Microsporidia spp.. The extrusion apparatus, composed of the coiled polar filaments and anchoring disc, are characteristic of this organism.11 The number and arrangement of the coils of the polar filament vary among genera and species.4 The polar filament is discharged through the anterior end of the spore, penetrating a new host cell and inoculating the infective sporoplasm. The spore has a thick wall with three distinct layers including a proteinaceous exospore, a chitinous endospore and a plasma membrane making it resistant in the environment. Initial histology on the liver of this crocodile showed almost all Kupffer cells contained numerous (often up to 20) phagocytosed, slightly refractile, oval to round organisms approximately 2-3 um in diameter. In addition, similar organisms were present within macrophages in the mesentery associated with moderate chronic inflammation and occasional organisms were present within splenic macrophages (identified by special stains). The organisms were positive for PAS, ZN and GMS stains.
Microsporidia are unicellular and spore forming obligate parasites which cannot grow & divide outside the host cell. They belong to the phylum Microsporia which has over 150 genera. These organisms were originally classified as fungi, reclassified as protozoa and then again as fungi. Microsporidia have chitin and trehalose which are fungal components, heat shock proteins similar to those of fungi and fungal α- and β-tubulins.11 All stages of the life-cycle occur within the parasitophorous vacuole. The most common microsporidia are: Enterocytozoon bieneusi, Encephalitozoon hellem, Encephalitozoon intestinalis (previously Septata intestinalis), and Encephalitozoon cuniculi.
As common pathogens of arthropods and fish, Microsporidia are responsible for economic losses in the fish farming and bee-keeping industries.11 Encephalitozoon cuniculi is the most commonly encountered microsporidial infection in veterinary practice. This infection was problematic for research rabbit colonies until screening for infection was introduced; however, it remains a common cause of fatal encephalitis in pet rabbits. E. cuniculi has also been described in farm-raised foxes and domestic dogs as well as spontaneous infection in numerous other domestic and wild species.11
Microsporidial infections have been described in numerous species of reptiles,10 most commonly bearded dragons which typically develop multisystemic granulomatous disease.9 Infection in crocodiles has not previously been described.10 Disseminated E. hellem infection has been described in a captive Egyptian fruit bat with the primary lesion being active cholangiohepatitis and acute renal tubular nephrosis associated with organisms.3 Microsporidial infection has been described in psitticine birds with histological changes ranging from hepatic necrosis in budgerigars associated with organisms1 to unilateral keraoconjunctivitis in a cockatoo.8
Multiple microsporidia have been identified as causative agents of disease in immunocompromised humans however Enterocytozoon bieneusi is the most commonly diagnosed.11
JPC Diagnosis: Granular leukocyte: Multiple Microsporidian spores, intracytoplasmic, freshwater crocodile (Crocodylus johnsoni), reptile.
Conference Comment: There are over 100 genera and 1000 species within the phylum Microsporidia which affect many invertebrates and all vertebrates, particularly if they are immunosuppressed.5 Microsporidians only reproduce asexually and are obligate intracellular eukaryotic organisms most closely related to fungi genomically. Infection follows injection of the sporoplasm into the host cell, where there is a proliferative merogenic phase, and eventually forming a sporont with a complex internal structure that lives within a parasitophorous vacuole. Spores are acid fast (carbol fuchsin), gram positive, luna positive, and contain a PAS positive polar granule.2
In reptiles, in addition to Encephalitozoon sp., Pleistophora sp. have been identified in lizards and snakes. In these populations of reptiles, organisms are identified intracytoplasmically within renal tubules, hepatocytes, alveolar epithelial cells, gastric mucosal epithelial cells, enterocytes, capillary endothelial cells, macrophages, and ventricular ependymal cells in the brain.5
Drs. LaDouceur and Murphy have recently identified microsporidian in peppermint shrimp (Lysmata spp.) which resulted in grossly swollen, opaque, pale tan nodules within skeletal musculature containing numerous microscopic microsporidian spores with minimal tissue inflammation.6
In fish, two common microsporidial infections are Loma salmonae and Pseudoloma neurophilia which cause pathologic changes in the gills and central nervous system, respectively. L. salmonae predominately affects salmonids and P. neurophilia commonly affects Zebrafish and neon tetra. In fact, P. neurophilia is the most common pathogen in zebrafish research facilities and results in emaciation and spinal curvature of affected animals giving credence to the common name “skinny disease”. Microscopically and often macroscopically, aggregates of microsporidia can be seen, and are termed “xenomas”; these cysts can be seen grossly on the gills of salmonids infected with L. salmonae and in the CNS of zebrafish infected with P. neurophilia.7
A common differential for Encephalitozoon spp., especially in mammals, is Toxoplasma gondii or Neospora caninum. Key differences are listed in the chart below.2
|
Toxoplasma spp. |
Encephalitozoon spp. |
|
Small cysts (60 µm) |
Pseudocysts are large (up to 120 µm) |
|
Spores not acid fast |
Spores are acid fast (Carbol fuchsin) |
|
Gram negative |
Gram positive |
|
Giemsa: cytoplasm is granulated |
Giemsa: cytoplasm is light blue |
|
Stains well with H&E |
Stains poorly with H&E |
|
Larger organism (2-6 µm) |
Smaller organism (1.5 x 2.5 µm) |
|
Tend to invoke necrosis |
Necrosis not a common finding |
|
Luna stain negative |
Luna stain positive |
Contributing Institution:
Gribbles Pathology
1868 Dandenong Rd
Clayton, Victoria 3168
Australia
References:
- Black SS, Steinohrt LA, Bertucci DC, Rogers LB, Didier ES. Encephalitozoon hellem in budgerigars (Melopsittacus undulatus).Vet Pathol. 1997;34(3):189-198.
- Cantile C, Youssef S. Nervous system. In: Maxie, MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 1. 6th ed. St. Louis, MO: Elsevier; 2016:385-386.
- Childs-Sanford SE, Garner MM, Raymond JT, Didier ES, Kollias GV. Disseminated microsporidiosis due to Encephalitozoon hellem in an Egyptian fruit bat (Rousettus aegyptiacus). J Comp Pathol. 2006;134(4):370-373.
- Franzen C, Müller A. Molecular techniques for detection, species differentiation, and phylogenetic analysis of Microsporidia. Microbiol. Rev. 1999;12:243-285.
- Jacobson ER. Parasites and parasitic diseases of reptiles. In: Jacobson ER, ed. Infectious Diseases and Pathology of Reptiles. Boca Raton, FL: CRC Press; 2007:580-581.
- LaDouceur EEB, Murphy BG. Microsporidiosis in peppermint shrimp (Decapoda: Hippolytidae: Lysmata ). J Zoo Wildl Med. 2017;48(4):1223-1229.
- Noga EJ. Fish Disease Diagnosis and Treatment. 2nd Ames, IA: Wiley-Blackwell; 2010:247-253.
- Phalen DN, Logan KS, Snowden KF. Encephalitozoon hellem infection as the cause of a unilateral chronic keratoconjunctivitis in an umbrella cockatoo (Cacatua alba). Vet Ophthalmol. 2006;9(1):59-63.
- Richter B, Csokai J, Graner I, Eisenberg T, Pantchev N, Eskens HU, Nedorost N. Encephalitozoonosis in two inland bearded dragons (Pogona vitticeps). J Comp Pathol. 2013;148(2-3):278-282.
- Scheelings TF, Slocombe RF, Crameri S, Hair S. Encephalitozoon hellem infection in a captive juvenile freshwater crocodile (Crocodylus johnstoni). J Comp Pathol. 2015;153(4):352-356.
- Wasson K, Peper RL. Mammalian Microsporidiosis. Vet Pathol. 2000;37:113-128.