Signalment:
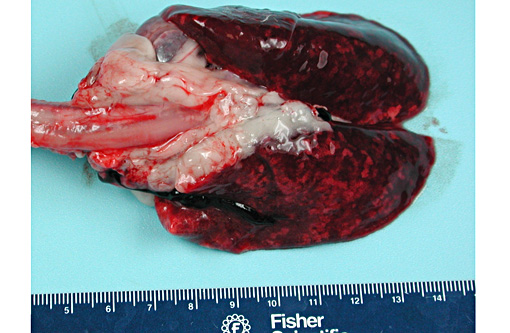
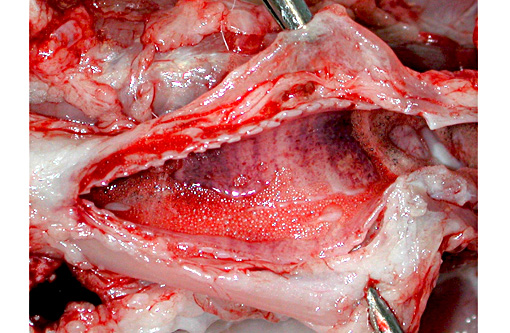
Gross Description:
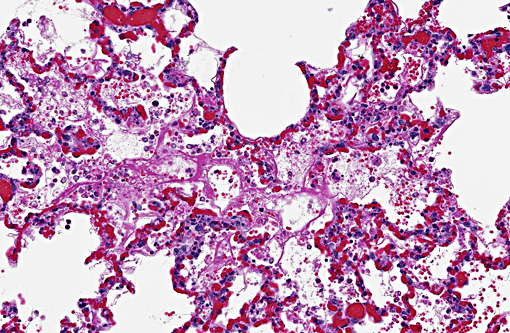
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Ricin is a glycoprotein composed of two glycoprotein chains (A and B) that are weakly linked by disulfide bonds. The B chain facilitates entry into the cell by binding to cell surface glycoproteins. The ricin A chain induces cellular necrosis by inhibition of protein synthesis through enzymatic alteration of the 28S ribosomal RNA loop contained within the 60S subunit.(1,3) The exotoxin, shiga toxin, produced by Shigella dysenteriae and Escherichia coli serotype O157:H7 have a similar mechanism of action.(7) Ricin also induces inflammation through the activation of mitogen activated protein kinases (MAPK), synthesis of proinflammatory RNA transcripts and production of increased levels of circulating cytokines and chemokines.(8)
All animals are potentially susceptible to toxicosis with ricin. The horse is the most sensitive domestic species. In contrast, sheep, cattle and pigs are more resistant while ducks and chickens are the most resistant. Cases of poisoning have resulted from the accident or deliberate introduction of beans or castor-cake with other feedstuffs. Mixture of feed in a plant previously used for castor seeds has even resulted in poisoning.(2)
The clinical signs and pathological changes in ricin intoxication are route specific.(3) The primary target of aerosolized ricin are the type I and II pneumocytes, and lesions following inhalation are primarily confined to the lungs and consist of intra-alveolar edema, acute alveolitis, and diffuse necrosis of the epithelium lining the lower respiratory tract.(1)
The gross lesions reported from experimental inhalation studies in rhesus macaques consist of focal hemorrhage in the intestines, brain, myocardium, and pleura.(7) Microscopic lesions consist of lymphocytic necrosis of lymphoid organs, hemorrhage, necrosis, hyaline droplets, and fibrin thrombi in the liver; hemorrhage and necrosis in the adrenal glands; tubular hyaline changes in the kidneys; degeneration and necrosis of the heart muscle; and congestion and hemorrhage of the gastrointestinal tract.(7)
Oral intoxication requires significantly more (up to 500X) material to reach toxic levels than by other routes of intoxication due to poor absorption in the digestive tract and possible enzymatic degradation in the digestive tract.(2,3) A review of 751 cases of castor bean ingestion in humans found a death rate of 1.9%.(3) The most common gross post-mortem findings reported in oral intoxication are multifocal ulceration and hemorrhages of the gastric and small intestinal mucosa. Findings in other organs systems are similar to parental introduction and include lymphoid necrosis in the mesenteric lymph nodes, gut-associated lymphoid tissue and spleen, as well as Kupffer cell and hepatocellular necrosis, diffuse nephritis and diffuse splenitis.(3)
In recent years, various extremist groups and individuals have used ricin in attacks or have been arrested for the possession of ricin. This is attributed to the ready availability of castor beans, ease of toxin extraction, and popularization on the Internet. None of these attacks resulted in human intoxications; however, they demonstrate that ricin is well known.(3,6)
The ricin toxin is capable of inducing the formation of antibodies since it is a protein. Immunity in cattle and calves has been demonstrated by feeding increasing amounts of the bean.(2) Currently, there is no licensed vaccine available for use in humans; however, one is under development, and molecules that have been evaluated include a deglycosylated ricin A chain, formalin-inactivated toxoid, and recombinant ricin A chain.(3,4) A vaccine candidate produced by the latter technique shows the most promise and is in more advanced stages of development in clinical trials.(3)
The research described herein was sponsored by Defense Threat Reduction Agency / Joint Science and Technology Office for Chemical Biological Defense, CBM.VAXBT.03.10.RD.P.011.
Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the U.S. Army.
JPC Diagnosis:
Conference Comment:
Aerogenous exposure is the most lethal form of ricin intoxication; however, a recent publication described two adult dogs that died 2-3 days following acute ingestion of fertilizer composed of residual castor bean plant material.(5) The dogs exhibited similar clinical signs as described in human ingestion cases, with vomiting and abundant hemorrhagic diarrhea due to ulcerative gastroenteritis. The most prominent histopathologic lesions were multifocal renal tubular degeneration and necrosis. Reports of such intoxication in dogs are rare, and only 9% ended in death or euthanasia in a retrospective review of 98 cases over a 11 year time period.(5)
References:
1. Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin poisoning: a comprehensive review. JAMA. Nov 9 2005;294(18):2342-2351.
2. Clarke EGC, Clarke ML. Poisonous plants. Garner's Veterinary Toxicology. Baltimore, MD: Williams and Wilkins Company; 1967:336-339.
3. Poli MA, Roy C, Huebner K, Franz DR, Jaax NK. Ricin. In: Dembek ZF, ed. Medical Aspects of Biological Warfare. Washington, DC: Borden Institute; 2007:323-335.
4. Reisler RB, Smith LA. The need for continued development of ricin countermeasures. Adv Prev Med. 2012;2012:149737.
5. Roels S, Coopman V, Vanhaelen P, Cordonnier J. Lethal ricine intoxication in two adult dogs: toxicologic and histopathologic findings. J Vet Diagn Invest. 2010;22(3):466-468.
6. Staff C. FBI searches for clues in ricin investigation http://www.cnn.com/2013/04/24/us/ricin-suspect-released/index.html?hpt=hp_inthenews. Accessed July 17, 2013.
7. Wilhelmsen CL, Pitt ML. Lesions of acute inhaled lethal ricin intoxication in rhesus monkeys. Vet Pathol. May 1996;33(3):296-302.
8. Wong J, Korcheva V, Jacoby DB, Magun B. Intrapulmonary delivery of ricin at high dosage triggers a systemic inflammatory response and glomerular damage. Am J Pathol. May 2007;170(5):1497-1510.